A Step-by-Step Guide to Making Octadecanol Powder from Rice Bran Wax
What Is Octadecanol?
Octacosanol, commonly known as Montany lalcohol, also known as Kontanyl alcohol. The chemical name for 1-Octacosanol or n-Octacosanol. Molecular formula C28H58 O, odorless and tasteless, relative molecular mass 410.77, non-hygroscopic, white scaly crystals or white powder, specific gravity 0.783g/cm3 (85 ℃), melting point 81 ℃ ~ 83 ℃, boiling point 250 ℃ / 0.4mmHg, insoluble in water, can be soluble in hot ethanol, ether, chloroform, benzene, toluene, methylene chloride, petroleum ether and other organic solvents. It is stable to acid, alkali and reducing agent, and is also stable to light and heat. Toxicological studies have shown that the safety of eicosanol is very high, the LD50 value of oral administration in mice is more than 18000mg/kg, and the sperm aberration test and Ames test in mice are all negative[1], which is much higher than the safety of table salt (LD50=3000mg/kg).
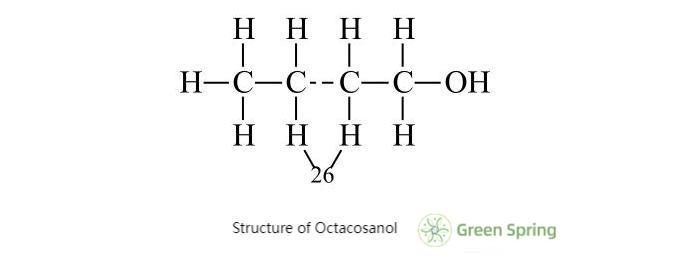
Octacosanol powder is a simple saturated straight chain alcohol composed of hydrophobic alkyl groups and hydrophilic hydroxyl groups. The hydroxyl group is the main site for various chemical reactions, such as esterification, halogenation, dehydration and hydroxylation, thiolation, etc. Its structure is shown below.
Distribution of Octacosanol
Octacosanol is found in nature mainly in the bound state (wax esters), with very little in the free state. Octacosanol was initially found in wheat germ and rice germ [2], and with further development of research, the lipids or epidermis of roots, stems, leaves, fruits, and seed kernels of many plants, as well as lipids secreted by insects, and the epidermis and viscera of animals, all contain Octacosanol. In addition, there are some scraps of grain and oil processing, such as rice bran wax, sugar cane wax, and other natural products. Its content is also high, and the current raw material for the production of Octacosanol Powder is mostly rice bran wax.

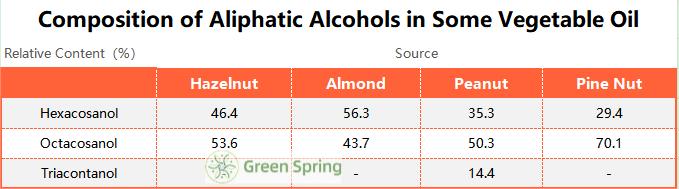
Vegetable fats and oils are the main sources of fatty alcohol. Peanuts, corn, rice, wheat, sugar cane, alfalfa, hazelnuts, pine nuts, sunflower seeds, raisins, etc. are rich in fatty alcohols, and at the same time have a large proportion of Octacosanol. The amount of Octacosanol in the total fatty alcohols of the germ, de-embryonated kernels, seed coat, hulls, and seeds of the above mentioned substances is usually more than 50% and even up to 70% in some cases. In addition, the flesh of fruits contains small amounts of Octacosanol, mainly in the peel wax. Higher levels of Octacosanol are also found in tomato skin wax. The composition of fatty alcohols in several vegetable oils is shown in Table 1.4, where it can be seen that Octacosanol is higher in pine nut oil, followed by hazelnut and peanut [3].

Table1. Composition of Aliphatic Alcohols in Some Vegetable Oil
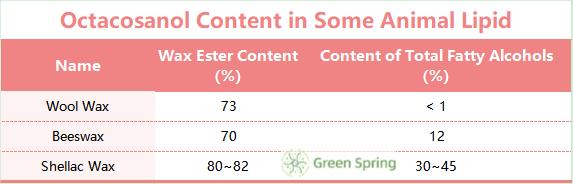
Existing studies have pointed out that Octacosanol and Triacontanol are contained in the fur, sebum and organ lipids of many animals, as well as in the waxes secreted by insects, such as white wax, beeswax and gum wax. The proportion of Octacosanol to total fatty alcohols in wool wax, beeswax and cordyceps wax, which are the most studied animal lipids, is shown in Table 2.
Table 2 Octacosanol Content in Some Animal Lipid
In summary, Octacosanol is usually combined with higher fatty acids to form esters in various animal and plant waxes, which are stable and low in content.
How to Make Octadecanol?
The extraction of Octacosanol powder is generally based on natural plants as raw materials and is separated from oils using methods such as dewaxing, solvent extraction, and supercritical CO₂ fluid extraction. Below is a step-by-step guide to making octadecanol powder from rice bran wax.

The solvent extraction method requires alcohol phase saponification, multiple solvent extractions, and then column chromatography or distillation for separation. To improve purity, repeated chromatography or distillation is often required. Finally, high purity products are obtained by using solvent based products.
Extraction of Octacosanol Powder from Rice Bran Wax
In the present experiment, the Octacosanol powder was prepared from refined furfury wax by a one-step process of calcification and saponification and a non-extraction process, which simplifies the existing saponification or ester exchange process, produces no wastewater, and reduces the cost of solvent consumption and energy consumption for solvent recovery. The effects of the optimal ratios of refined furfury wax, calcium hydroxide and water, saponification temperature and saponification time on the saponification and extraction process were investigated. The results showed that the refined furfury wax: calcium hydroxide: water 10:2:3, saponification temperature 85 ℃, saponification time 6 h for the optimal saponification reaction conditions, the optimal temperature of column molecular distillation for 190 ℃ and 210 ℃, respectively, the quality fraction of octadecanol powder is 93%, the mass fraction of tricosanoids is 95%, in line with the needs of the pharmaceutical and food industries.
1 Materials and Methods
1.1 Experimental Materials
Commercial rice bran wax, eicosanol sample, calcium hydroxide, hydrochloric acid, anhydrous ethanol, acetone, etc. were analytically pure.
DK2S26 digital display electric thermostatic water bath, SD202-0 (AS) electric thermostatic blower drying oven, WH220 heating magnetic stirrer, 7890A gas chromatograph, RV-10 rotary evaporator, and other equipment are commonly used in the laboratory.
1.2 Experimental method
1.2.1 One-Step Calcium Saponification Preparation Process
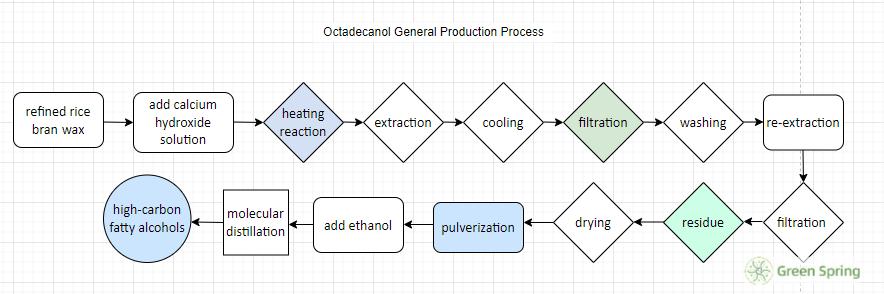
The general production process is complex, 1 T of refined furfury wax calcium soap discharged wastewater up to 10 T, of which the COD reaches more than 3,000, pH value of 10 or more. Octadecanol general production process: refined rice bran wax → add calcium hydroxide solution → heating reaction → extraction → cooling → filtration → washing → re-extraction → filtration → residue → drying → pulverization → add ethanol → molecular distillation → high-carbon fatty alcohols.
This experiment involves a one-step process of calcification and saponification, as well as a non-extraction process. Due to the small amount of water added, it solves the problem of complex steps in existing saponification or ester exchange processes, resulting in a large amount of high concentration organic wastewater. It reduces the organic solvent extraction process, reduces solvent consumption costs, and energy consumption for solvent recovery. The one-step production process of calcification and saponification: refined bran wax → addition of calcium hydroxide solution → heating reaction → drying → crushing → addition of ethanol → pre fractionation → molecular distillation → high carbon fatty alcohols.
1.2.2 Optimization of Calcium Saponification Process Conditions
The one-step preparation process of calcium saponification was adopted in the experiment, and the effects of the optimal ratio of furfury wax, calcium hydroxide and water, the amount of calcium hydroxide, the saponification temperature and the saponification time on the saponification rate were investigated separately. Weighing 100 g of refined furfury wax, added to a 500 mL beaker, 20 g of calcium hydroxide and 20 mL of water were added to the furfury wax, heated and stirred constantly to raise the temperature to 90 ℃ for 8 h. After the saponification reaction was completed, 3 drops of phenolphthalein ethanol solution (1% by volume) were added into the saponified product as an indicator, and the saponified product was titrated with a standard hydrochloric acid solution (0.5 mol/L) immediately, and the titer was set at 0.5 mol/L. The titer was set at 0.5 mol/L, and the titer was set at 0.5 mol/L. The saponified product became colorless when it was purplish red. When the purple-red color becomes colorless, the endpoint of the titration is reached, and the saponification rate is calculated by reading the volume of the consumed standard hydrochloric acid solution [4].
1.2.3 Purification by Molecular Distillation
The above saponified furfury wax was dried, cooled and crushed to 50~100 mesh as raw material, and 15 parts of about 95% ethanol solution were added, and purified by column molecular distillation technique, with the vacuum degree of 0.5~5 Pa, the temperature of the evaporating surface of 110~210 ℃, the temperature of the condensing surface of normal temperature, the stirring speed of 100~600 r / min, and the heating temperature adopting the method of intermittent rise, and holding the temperature for 30 min every 20 ℃ until the temperature rises to 230 ℃, and the total reaction time was controlled to 30 ℃. When the temperature rises to 50 ℃, hold the temperature for 30 min, and then hold the temperature for 30 min every 20 ℃ until the temperature rises to 230 ℃, and the total reaction time is controlled at 30 h. The Octacosanol is prepared by using the surface accumulation method. The Octacosanol was measured by the area normalization method.
2 Results and Analysis
2.1 Influence of the Amount of Water on the Saponification Rate
Experimental results are shown in Figure 2, weighing 100 g of refined furfural wax into a 500 mL beaker, 20 g of calcium hydroxide and 10-40 mL of water were mixed and added to the furfural wax, heated and stirred constantly, so that the temperature was raised to 90 ℃ under the extraction conditions for 8 h, the saponification rate increased with the increase in the amount of water, the increase in the rate of saponification was limited when the amount of water exceeded 30 mL, and the optimum amount of water was determined to be 30 mL, which is the optimum amount for the saponification rate and the cost of drying. The optimal amount of water was determined to be 30 mL.
Figure 2 Effect of Calcium Saponification Process Conditions on Saponification Rate

2.2 Effect of Calcium Hydroxide on Saponification Rate
The results are shown in Fig. 2. 100 g of refined furfural wax was added into a 500 mL beaker, 10-40 g of calcium hydroxide and 30 mL of water were added into the furfural wax, and the saponification rate increased with the increase of calcium hydroxide under the extraction condition of 8 h. The optimal amount of calcium hydroxide was 20 g. The increase in saponification rate was not obvious after the increase of calcium hydroxide. The optimum dosage of calcium hydroxide was determined to be 20g.
2.3 Effect of Saponification Time on Saponification Rate
The experimental results are shown in Figure 2, weighing 100 g of refined furfural wax into a 500 mL beaker, 20 g of calcium hydroxide and 30 mL of water were added to the furfural wax after mixing, heated and stirred constantly, so that the temperature was raised to 90 ℃ The saponification rate increased with the increase of time under the extraction conditions of 5-9 h. When the reaction time was more than 6 h, the increase of saponification rate became very slow, and the optimum saponification time was determined to be 6 h. The saponification time was determined to be 6 h. The saponification time was determined to be 6 h. Therefore, the optimal saponification time was determined to be 6 h.
2.4 Effect of Saponification Temperature on Saponification Rate
Experimental results as shown in Figure 2, weighing 100 g of refined furfural wax added to 500 mL beaker, 20 g of calcium hydroxide and 30 mL of water mixed and added to the furfural wax, heating and stirring, so that the temperature rises to 80 ~ 100 ℃ saponification reaction for 6 h under the conditions of extraction, the saponification rate with the rise in temperature and increase, when the reaction temperature reaches 85 ℃ after the increase in saponification rate is not obvious, 90 ℃ after the contrary, a slight decline, the temperature is too high, resulting in the decomposition of saponin, so the optimal reaction temperature was determined to be 85 ℃, 75% saponification rate. The saponification rate increased as the temperature increased, when the reaction temperature reached 85 ℃ saponification rate did not increase significantly, after reaching 90 ℃ on the contrary, there was a slight decline in the high temperature led to the decomposition of saponin, so the optimal reaction temperature was determined to be 85 ℃, saponification rate of 75%.
2.5 The Effect of Distillation Temperature on the Purification of High-Carbon Fatty Alcohols
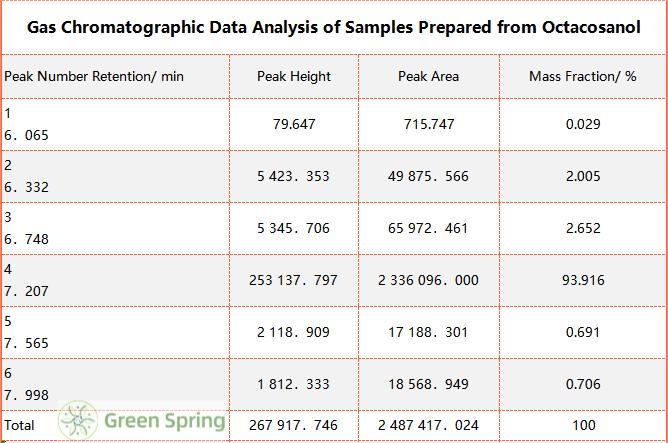
The effect of distillation temperature on the separation effect is very significant [5], according to the 1.2.3 molecular distillation purification production process conditions, the only change in the distillation temperature 180 ~ 230 ℃, the experimental results are shown in Fig. 3, Fig. 4, Fig. 5, and Table 1, Table 2, when the column molecular distillation temperature reaches 190 ℃ when the mass fraction of cis-octadecanol reaches a maximum of 93%. When the column molecular distillation temperature reached 190 ℃, the mass fraction of cis-octadecanol reached 93%.
Figure 3 Effect of Distillation Temperature on the Mass Fraction of High Carbon Fatty Alkanols
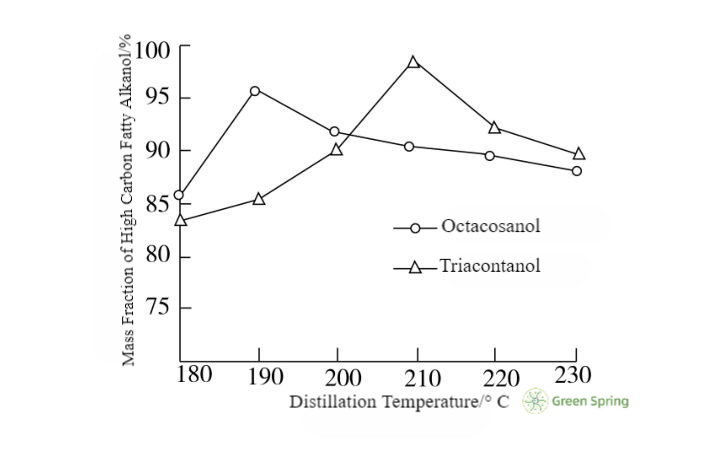
Figure 4 Gas Chromatographic Detection of Octacosanol Standard
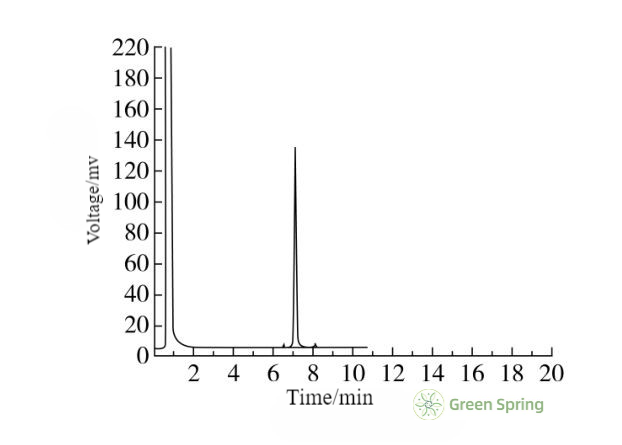
Table 3 Gas Chromatographic Data Analysis of Octacosanol Standard
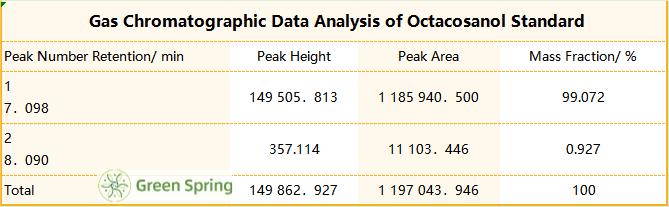
Figure 5 Gas Chromatographic Detection of Samples Prepared from Octacosanol
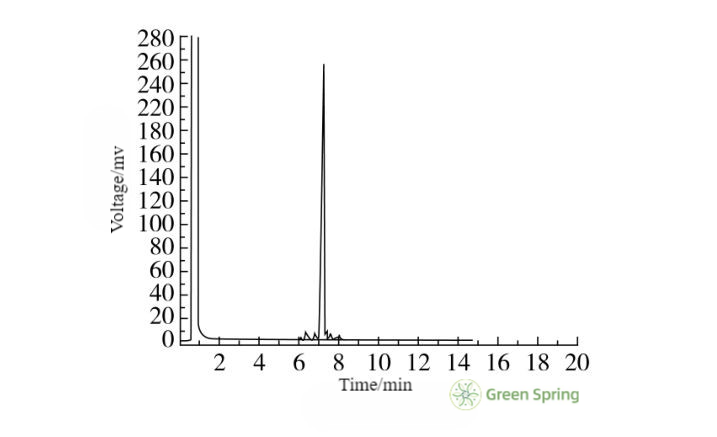
Table 4 Gas Chromatographic Data Analysis of Samples Prepared from Octacosanol
3 Conclusion
Experimental results show that the refined furfury wax: calcium hydroxide: water for 10:2:3, saponification temperature 85 ℃, saponification time 6 h for the optimal saponification reaction conditions, the saponification rate of 75%, the optimal temperature of column molecular distillation were 190, 210 ℃. The mass fraction of octadecanol powder was 93%, and the mass fraction of tertiary alkanol was 95%. The one-step calcium saponification preparation process greatly reduces the number of process steps, no wastewater generation, and is conducive to the realization of large-scale industrial production.
Reference:
[1] Wang Cunyan, Fan Tao, Wu Chuanhua, Et Al. Research Progress of Octacosanol and Its Development and Application in Food[j]. Industry Review, 2007 , 5(2): 14-16
[2] Li Qinmei. Progress of Extraction and Detection Methods of Octacosanol[J] . Journal of Food Safety and Quality Testing, 2013 , 4(1):279-282
[3] Chen Fang. Extraction and Refining of Octacosanol from Rice Bran and Research on Its Anti-Fatigue Function [d]. Beijing: China Agricultural University, 2003, 9(3): 1-122
[4] Yang Hao, Li Lilong, Wu Xin, Et Al. Study on the Preparation Process of Octacosanol in Beeswax[J]. Journal of Jiangxi Agricultural University, 2012, 35(5) : 1053 -1057
[5] Gu Zhiwei, Mao Jia, Huang Shaolie. Preparation of Eicosanoids from Rice Bran Wax Under Ultrasonic Conditions[J]. China Fats & Oils, 2008, 33(6) : 54-56


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




