Green Spring Technology: A Fully Traceable and Consistent Full-Range Glutathione Raw Material Solution
Part I: Challenges in Glutathione Raw Materials: Why “Traceability” and “Consistency” Matter Most?
Glutathione, a vital antioxidant in the human body, is expanding its applications in pharmaceuticals, health supplements, functional foods, and premium cosmetics at an unprecedented pace. Selecting high-quality glutathione raw materials is the cornerstone for creating effective and safe end products.
Yet behind this thriving market, the complexity and uncertainty of the raw material supply chain have become a core bottleneck for many enterprises:
■ “Black box” supply chains: Uncontrollable risks?
When your raw material sources are unclear and production environments/processes untraceable, any oversight—from starting materials to manufacturing—can introduce unknown risks, directly threatening your final product's safety and compliance.
■Batch quality fluctuations causing inconsistent product efficacy?
Significant variations in key metrics like purity, impurity levels, and solubility across different raw material batches lead to erratic product performance. This not only damages brand reputation but may also trigger market disputes.
■ Missing technical documentation hindering registration applications?
Particularly in pharmaceuticals and health supplements, lacking comprehensive, compliant technical files (e.g., DMFs, complete COAs) and verifiable traceability data will make product registration an uphill battle.
These are not merely “procurement issues”—they are strategic core concerns affecting your brand credibility, market competitiveness, and long-term development.
Facing this industry-wide challenge, Green Spring Technology offers a fundamental solution: We firmly believe true quality begins with “traceability” and is achieved through “high consistency.”
Green Spring Technology is committed to transforming your supply chain from a “black box” to a “transparent box” through our full-series glutathione raw material solutions featuring end-to-end traceability and batch-to-batch consistency. This provides a solid foundation for your product's excellence and reliability, starting from the very source.
Part II: What is Glutathione?
Glutathione (chemical name: γ-glutamylcysteinylglycine) is a natural tripeptide found in living organisms. It is formed by the unique peptide bonds connecting three amino acids: glutamic acid, cysteine, and glycine.
Hailed as the “master antioxidant” within cells, it is the core molecule responsible for maintaining the body's redox balance and protecting cells from damage. High concentrations of glutathione are present in nearly all human cells, particularly in the liver—the body's primary detoxification organ.
The key point: Glutathione primarily exists in two forms within the body, and its dynamic equilibrium directly determines its biological activity:
Form
| Reduced Glutathione
| Oxidized Glutathione
|
Chemical Structure
| -SH (Mercaptan/Thiol Group), Active
| -S-S- (Disulfide Bond), Inactive
|
Biological Activity
| Directly bioactive, serving as the primary agent for antioxidant and detoxification functions.
| No direct biological activity; it is the oxidized product of reduced glutathione after it has performed its functions.
|
Functional Role
| “Warrior”: Directly neutralizes free radicals.
| “Reserve/Recycling Unit”: Can be re-reduced to its active form by enzymes within the body.
|
Application Status
| Preferred for exogenous supplementation. Our core objective is to elevate reduced glutathione levels within the human body.
| Typically not used as a direct supplement.
|
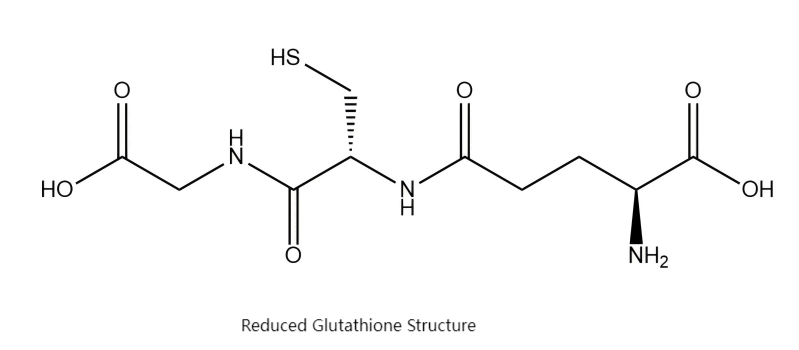
Green Spring Technology Insights:
Understanding the pivotal role of reduced glutathione is the first step in selecting premium raw materials. However, its inherent thiol groups are highly reactive and prone to oxidation, imposing stringent demands on production processes, purification techniques, and storage conditions. Maximizing the stability and activity of reduced glutathione throughout the supply chain serves as the primary benchmark for evaluating a raw material supplier's technical capabilities.
Part III Glutathione's Mechanism of Action
Glutathione's formidable efficacy stems from its intricate, multifaceted mechanisms within cells. Acting as a versatile agent, it constructs a three-dimensional defense and regeneration system.
This system operates not in isolation but as a dynamically balanced, cyclically regenerating entity. The ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) serves as a critical indicator of the body's oxidative stress levels and cellular health status. Maintaining high GSH levels and a healthy GSH/GSSG ratio is essential for ensuring the proper functioning of all these processes.
Green Spring Technology Perspective:
The efficacy of exogenous glutathione supplementation depends not only on its initial purity and form (reduced), but also on its bioavailability. The physicochemical properties of the raw material—such as particle size, solubility, and stability—directly impact its activity within the product and the efficiency of its absorption and utilization by the human body. Therefore, “high purity” and “exceptional stability” are not merely quality indicators; they are guarantees of efficacy. This is precisely the core value Green Spring Technology delivers through rigorous production processes and quality control—ensuring every gram of raw material you use maximizes its conversion into tangible efficacy in the final product.
Part IV: Diverse Applications of Glutathione Raw Materials
As a vital bioactive ingredient, glutathione possesses unique physicochemical properties that enable its broad application across multiple industrial sectors. Green Spring Technology is committed to supplying glutathione raw materials that meet the specific industry standards and regulatory requirements of clients across various sectors.
1. Pharmaceutical Industry
· Application Overview: Glutathione serves as a key active pharmaceutical ingredient (API) or intermediate in drug manufacturing.
· Green Spring Technology Solution: We provide pharmaceutical-grade glutathione produced under strict adherence to cGMP standards. Our raw materials feature complete Drug Master Files (DMFs) and fully traceable quality records. Their exceptional batch-to-batch consistency meets the stringent requirements for ingredient uniformity and stability in pharmaceutical formulation production, supporting clients' global registration submissions.

2. Dietary Supplements and Nutritional Health Products
· Application Overview: Glutathione is widely used in health products such as dietary supplements and nutritionally formulated foods.
· Green Spring Technology Solution: We provide food-grade glutathione raw materials in multiple specifications suitable for developing various dosage forms including tablets, hard capsules, and powders. We strictly control physical properties like particle size and flowability to ensure excellent processing adaptability in end-product manufacturing.
3. Cosmetics and Personal Care Products
· Application Overview: In cosmetics, glutathione serves as an ingredient for formulation development.
· Green Spring Technology Solution: We prioritize critical performance metrics for cosmetic applications, offering high-purity, color-stable grades. Consistent physical and chemical properties across batches help maintain your product's color, texture, and overall stability.

4. Animal Nutrition & Health
· Application Overview: Glutathione serves as a feed additive or premix component in animal nutrition.
· Green Spring Technology Solution: We provide cost-effective glutathione products compliant with relevant feed regulations, supporting customer innovation and development in animal health management.
Green Spring Technology's Cross-Industry Support:
From pharmaceutical manufacturing to consumer goods, diverse industries share core requirements for regulatory compliance, quality consistency, and supply chain reliability. Green Spring Technology's “Traceable and Consistent” quality system ensures our glutathione raw materials serve as a dependable, trustworthy component in your supply chain, laying a solid foundation for your product success.
Part V: How to Select High-Quality Glutathione Raw Material Suppliers: A Professional Evaluation Framework
Choosing a reliable, professional glutathione raw material partner is crucial for ensuring your project's smooth progress and market success. Faced with numerous suppliers, price alone is insufficient for optimal decision-making. We recommend a comprehensive evaluation across five core dimensions to ensure your chosen supplier meets both current and future strategic needs.
1. Compliance and Quality Systems
· Key Question: Does the supplier hold internationally recognized quality management system certifications (e.g., GMP, ISO9001)? Do their production facilities and processes comply with regulatory requirements of your target markets (e.g., China NMPA, US FDA, European EDQM)?
· Evaluation Value: This represents the baseline and commitment of the supplier's quality management, serving as the fundamental guarantee for product safety and legal compliance.
2. Traceability Capability and Data Integrity
· Key Questions: Can the supplier provide a fully traceable, verifiable chain of records from starting materials to finished product release? Can they furnish complete, accurate Certificate of Analysis (COA) for each batch?
· Evaluation Value: A comprehensive traceability system and data integrity are not only essential for audits and registrations but also enable rapid, precise root cause analysis during incidents, minimizing risks.

3. Batch Consistency and Stability Data
· Key Question: Can the supplier provide historical batch testing data demonstrating exceptional batch-to-batch consistency for critical parameters (e.g., purity, related substances, heavy metals, microbial limits)? Is there stability study data supporting the product's shelf life and storage conditions?
· Evaluation Value: Minimal batch-to-batch variation ensures more stable end-product quality, reduces process adjustments, and boosts production efficiency—directly impacting your brand reputation and manufacturing costs.
4. Technical Documentation and Regulatory Support
· Key Question: Can the supplier provide comprehensive technical documentation required for product registration, including detailed process descriptions, impurity profiling analyses, and physicochemical property studies? Have they prepared or submitted registration filings?
· Evaluation Value: Complete registration support documentation significantly accelerates your product's time-to-market while reducing your own regulatory compliance burden and risk.
5. Technical Collaboration and Customization Capabilities
· Key Question: Does the supplier possess robust R&D capabilities to understand your specific application scenarios and provide necessary technical parameter support? Can they offer flexible customization services based on your unique requirements (e.g., specific particle size, packaging specifications)?
· Evaluation Value: This determines whether the supplier is merely an order executor or a strategic partner capable of growing alongside you and resolving complex technical challenges.
Green Spring Technology's Commitment:
We understand your supplier evaluations are rigorous and comprehensive. Green Spring Technology welcomes your careful assessment based on the above framework. We believe our continuous investments in end-to-end traceability systems, data-driven batch consistency control, and comprehensive regulatory documentation support provide the optimal response to your core concerns.
Part VI: GreenSpring Technologies' End-to-End Traceable Glutathione Solution
GreenSpring Technologies has established an end-to-end quality solution built on the cornerstones of “traceability” and “high consistency.” We not only offer a diversified product portfolio but also ensure every product embodies our commitment to safety, compliance, and exceptional quality.
Full Product Range for Precise Application Needs
We understand that different application fields have distinct specifications for glutathione. To address this, Green Spring Technology provides a rigorously differentiated product line, ensuring you access the most suitable raw material options for your industry.
Product Name
| Key Specifications and Standards
| Primary Applications
|
| High-purity reduced form, compliant with food/dietary supplement standards
| Dietary supplements, functional foods, and general nutritional health products
|
| High-purity acetylated specification with enhanced chemical stability
| Premium dietary supplements, functional foods for specific research applications
|
| Strictly compliant with food safety regulations
| Food fortification, nutritional formula foods
|
| Focused on low impurity control, color consistency, and batch stability
| Formulations for whitening, anti-aging skincare, and personal care products
|
Pharmaceutical-grade glutathione
| Compliant with cGMP, highest purity, supports DMF/CEP filing
| Pharmaceutical formulations, export-grade premium applications
|
Our Core Strength: Transforming Commitments into Verifiable Actions
1. Cutting-Edge Manufacturing Processes and Consistency Assurance
· We employ precision-controlled microbial fermentation and purification technologies to ensure high purity and low impurity levels from the source.
· Through fully automated production systems and strict standardized operating procedures, we monitor and record every critical process parameter in real-time. This fundamentally guarantees exceptional batch-to-batch consistency, making your production processes more stable and efficient.
2. Rigorous Quality Systems and Compliance Documentation
· Our production facilities operate under cGMP and ISO9001 quality systems, ensuring every stage from raw materials to finished products remains under strict control.
· We provide detailed, compliant analytical reports for every batch and prepare or have submitted complete Drug Master Files (DMFs) for pharmaceutical-grade clients, fully supporting your product's global regulatory registration.

3. Comprehensive Traceability System
· We have established a fully digital traceability system covering critical starting materials, production batches, intermediate product inspections, and final product release. This is not merely a concept but an auditable operational practice with complete documentation, ensuring rapid identification and isolation of any issues to significantly reduce your supply chain risks.
4. Expert-Level Technical Collaboration
· We deliver not just products, but in-depth services built upon them. Our technical team provides tailored support—including relevant technical parameters and stability study data—based on your specific applications, acting as an extension of your R&D capabilities to optimize formulations and processes.
Green Spring Technology's Commitment:
We are dedicated to making “traceability” and “consistency” tangible, stable values you can experience through audits, data, and long-term collaboration—not just marketing slogans. Choosing Green Spring Technology means selecting a supply chain partner that reduces compliance risks, enhances end-product competitiveness, and earns your lasting trust.
Part VII: Frequently Asked Questions
We've compiled the most frequently asked questions from clients during evaluation and partnership discussions to provide clear answers. For specific inquiries, please contact us anytime.
Q1: How should S-Acetyl-L-Glutathione and conventional yeast-derived reduced glutathione be selected for application?
A: These products address distinct technical needs, primarily differing in chemical stability and application focus.
· Yeast-derived Glutathione (Reduced) is a naturally occurring form extracted through biological fermentation. It is widely used in conventional dietary supplements, functional foods, and nutritional products, featuring mature technology and broad applicability.
· S-Acetyl-L-Glutathione is a stabilized form modified by acetylation. Its molecular structure provides enhanced stability in gastrointestinal environments and product formulations. It is frequently used in premium supplements with stringent ingredient stability requirements or in functional food development for specific research directions. Our technical team can provide detailed selection recommendations based on your specific product design and positioning.
Q2: We plan to export our products. Can your company provide the full set of compliance documents required for product registration?
A: Yes. This is part of our core strengths. For different markets and applications, we can provide foundational documents including comprehensive and accurate Certificate of Analysis (COA) and Material Safety Data Sheets (MSDS). For pharmaceutical applications, we can offer open reference to Drug Master Files (DMF) or relevant technical support documents for qualified clients, fully assisting you in meeting regulatory registration requirements for major markets such as China's NMPA, the US FDA, and Europe's EDQM.
Q3: Can samples be provided for preliminary testing and evaluation?
A: Certainly. We encourage clients to verify our quality through physical samples. We can supply small-batch samples to qualified potential partners for your preliminary R&D, pilot testing, or quality assessment. Please submit your request via the contact information at the end of this document, and our sales engineers will assist you.
Q4: What are the key differences in quality control between different product grades (e.g., food-grade and pharmaceutical-grade)?
A: The primary distinctions lie in the rigor of quality control standards and the specific impurity profiles targeted.
· Pharmaceutical-grade materials adhere to the highest pharmacopoeial standards (e.g., USP/EP), enforcing stringent controls over all known and unknown impurities, heavy metals, residual solvents, and microbial limits. Production must occur under cGMP conditions to ensure full traceability.
· Food-grade/Cosmetic-grade materials strictly adhere to their respective industry regulations and safety standards. While ensuring safety, greater emphasis is placed on indicators affecting product physical properties (e.g., color, solubility) and processing performance. Regardless of grade, we follow a unified, rigorous quality management system to ensure product safety and reliability.
Q5: What are your Minimum Order Quantity (MOQ) and lead time?
A: To accommodate diverse customer needs, we maintain flexible MOQ policies, with specific quantities varying by product specifications and packaging requirements. Standard products typically ship within 2-4 weeks after contract signing. For bulk orders or customized requests, our sales team will provide precise MOQ details and delivery schedules.
Part VIII: Contact Us
Launching Reliable Partnerships: Turn Traceability and Consistency into Your Supply Chain Advantage
Whether you're in the initial technical exploration phase or ready to find a reliable raw material partner for your next exceptional product, GreenSpring Technology's professional team stands ready to provide comprehensive support from technical matching to commercial implementation.
We look forward to offering you:
· Detailed product specifications and compliance documentation
· Expert raw material selection recommendations tailored to your application
· Highly competitive sample and quotation solutions
· Dedicated one-on-one technical and order support
Contact GreenSpring Technology Today
We understand that reliable partnerships begin with engaging a responsible team. As your dedicated contact, I will ensure your questions are promptly addressed, your requirements are accurately conveyed, and coordinate end-to-end services from technical support to logistics.
Contact Information:
· Company Address: Room 604, Building A, National Digital Publishing Base, Tiangu 7th Road, High-Tech Zone, Xi'an, Shaanxi Province, China 710076
· Company Phone: +86 29 88313578
· Mobile/WhatsApp: +86 13649243917 (Recommended for fastest response)
· Email: helen@greenspringbio.com
· Official Website: https://www.greenspringnatural.com/
-
Prev
One-Stop Solutions for Standardized Artichoke Extract Ingredients | Green Spring Technology
-
Next
Powering Natural Antimicrobial Formulations with 95% Rutin API from Green Spring Technology


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




