Natural Xanthohumol in Hops Extract Enhances Bone Health Product Upgrades
With the growing emphasis on healthy living, modern consumers are increasingly prioritizing bone nutrition and daily care. Among numerous natural plant-derived active ingredients, xanthohumol—a compound sourced from hops—has emerged as a focal point, garnering significant attention from both research and industry sectors.
Xanthohumol is a unique natural flavonoid compound found exclusively in hops. It possesses excellent antioxidant properties and demonstrates promising potential in maintaining cellular health. Research indicates this compound helps support osteoblast activity while regulating osteoclast activity, offering new avenues for developing bone-related dietary supplements and functional foods. Currently, multiple international patents have been secured for xanthohumol applications, indicating broad industrialization prospects.
Adhering to the philosophy of “Natural Science, Healthy Future,” Green Spring Technology now introduces high-purity, premium-quality xanthohumol powder extracted from hops. We are committed to providing stable, reliable natural ingredient solutions for global clients, empowering brands to develop more innovative and market-competitive bone nutrition products, and jointly advancing the health industry's upgrade.
1 Green Spring Technology High-Purity Xanthohumol: Multi-Pathway Regulation of Bone Metabolism and Cell Differentiation—An Innovative Raw Material Solution Backed by In Vitro Evidence
Xanthohumol, a flavonoid compound derived from hops, demonstrates distinct in vitro activity in regulating bone metabolism. Its multi-target mechanism influences osteoblast and osteoclast differentiation processes, demonstrating development potential as a functional bone nutrition ingredient.
At the molecular level, xanthohumol significantly upregulates Runx2 expression and promotes differentiation markers (e.g., ALP, osteocalcin) in osteoblasts. Simultaneously, it inhibits adipogenesis by suppressing the PPARγ signaling pathway, thereby promoting mesenchymal stem cell differentiation toward osteogenic differentiation. Regarding antioxidant effects, anthohumol activates the Nrf2-ARE pathway to enhance intracellular antioxidant enzyme expression (e.g., HO-1, NQO1), mitigating oxidative stress impacts on osteoblast activity.
In osteoclast regulation, anthohumol inhibits RANKL-induced NF-κB activation by reducing IκBα degradation and p65 nuclear translocation. Simultaneously, it inhibits Ca²⁺ oscillations and the NFATc1 signaling pathway, downregulating key osteoclast differentiation markers (e.g., TRAP, CTSK). MAPK pathway studies indicate that anthoxymol suppresses ERK, p38, and JNK phosphorylation, thereby affecting c-Fos and NFATc1 transcriptional activity.
Green Spring Technology employs proprietary extraction processes to provide epigallocatechin gallate (EGCG) raw material with >98% purity, featuring distinct HPLC fingerprint profiles and batch consistency. This material exhibits excellent stability in both aqueous and lipid-soluble systems, making it suitable for developing various dosage forms such as solid beverages and soft capsules. We provide comprehensive in vitro cellular experimental data support, including osteoblast differentiation assays, osteoclast formation inhibition studies, and antioxidant activity measurements, serving as a reference for product development.
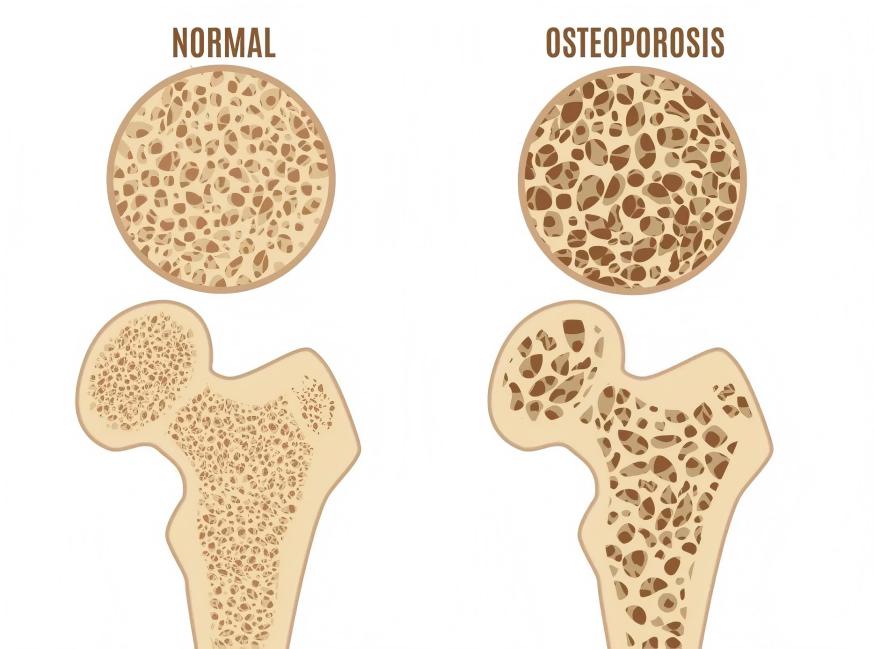
2 Green Spring Technology Xanthohumol: Multi-Pathway Regulation of Cellular Function, Empowering Next-Generation Bone Nutrition Innovation
Xanthohumol, a natural bioactive compound derived from hops, has garnered significant attention in the field of bone nutrition in recent years. Its multi-targeted properties in cellular signaling and metabolic regulation offer new avenues for developing innovative bone nutrition products.
Research indicates that xanthohumol interacts with multiple cellular signaling pathways to maintain the healthy equilibrium of bone tissue. By influencing RANKL-mediated cellular signaling, it participates in regulating various cellular functional pathways including NF-κB, Ca²⁺/NFATc1, and MAPK, thereby supporting bone health at the molecular level.
At the mechanistic level, p-Cresol has been found to regulate IκB protein metabolism, influencing NF-κB's intracellular localization. Simultaneously, it modulates Ca²⁺ signaling frequency, positively affecting NFATc1 activity. Furthermore, p-Cresol exhibits regulatory effects on EPK1/2 and c-fos expression within the MAPK pathway. These multifaceted mechanisms provide a scientific rationale for p-coumaric acid's application in bone nutrition.
Green Spring Technology employs innovative extraction processes to produce high-purity, highly stable p-coumaric acid raw materials. Our products exhibit excellent biocompatibility and compatibility, making them suitable for dietary supplements, functional foods, and specialized dietary products. We are committed to providing partners with premium natural raw material solutions, jointly advancing the development of bone nutrition products toward greater efficiency and innovation.
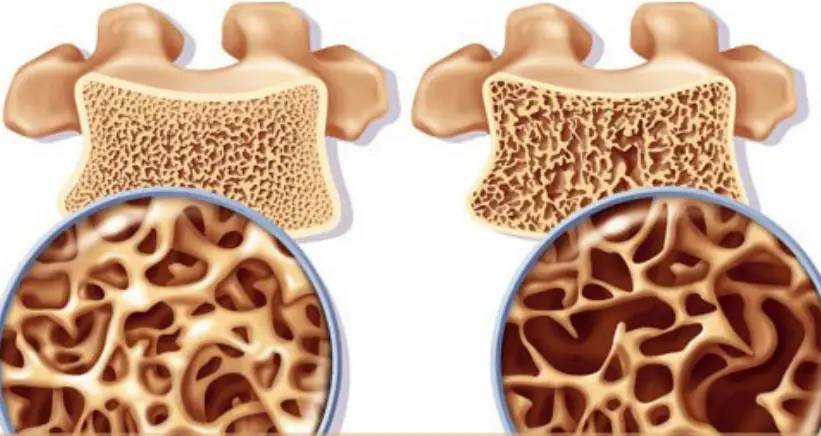
3 Innovative Bone Nutrition Solutions: Green Spring Technology's High-Purity Xanthohumol Powder, Empowering Product Upgrades
As modern society increasingly prioritizes healthy aging, the bone nutrition sector faces renewed demand for product innovation. Xanthohumol—a bioactive flavonoid from hops—has demonstrated multi-pathway potential in regulating bone metabolism through extensive cellular studies, opening new avenues for advanced bone health products.
Green Spring Technology leverages advanced extraction and purification techniques to introduce high-purity, high-stability xanthohumol powder with the following core advantages:
✨ Purity >98%, backed by complete HPLC detection spectra and a rigorous quality control system ensuring batch-to-batch consistency;
✨ Excellent bioavailability and compatibility, suitable for diverse formulations including solid beverages, soft capsules, and tablets;
✨ Comprehensive in vitro cellular experimental data support, including multi-dimensional research outcomes on osteogenic differentiation, antioxidant activity, and cellular metabolism.
Learn More About Our One-Stop Solution for Standardized Hop Extract Ingredient.
We are committed to providing partners with scientifically validated, commercially valuable raw material solutions that help clients:
· Break through the homogenized competition of traditional bone nutrition products by creating innovative offerings with differentiated competitive advantages;
· Gain end-to-end technical support spanning ingredient mechanism research to product development;
· Accelerate product R&D cycles to swiftly respond to market demands.
Green Spring Technology collaborates with industry partners to leverage natural science and technology, jointly advancing the professionalization and scientific upgrading of bone health products. We deliver more effective and safer nutritional solutions for consumers.
Contact us at helen@greenspringbio.com or WhatsApp: +86 13649243917 for complimentary samples and technical documentation.
References
[1] Xu Lijing, Chen Mingsheng, Si Lei. Current Status, Challenges, and Recommendations for the Health Economic Evaluation of Osteoporosis [J]. Chinese Journal of Endocrinology and Metabolism, 2021,
[2] Wang Wenhui. The Effects and Advances of Antiosteoporosis Drugs [J]. Chinese Journal of Medicine, 2021, 56(11): 1189-1192.
[3] Lin Canbin. Clinical Efficacy of Different Types of Bisphosphonate Drugs in the Treatment of Osteoporosis [J]. Journal of Rational Drug Use, 2021, 14(32): 109-111.
[4] Yuan Chunyan, Ren Mulian. Sex Hormone Therapy for Postmenopausal Osteoporosis [J]. Practical Journal of Obstetrics and Gynecology, 2020, 36(7): 494-497.
[5] Qu Hangshuai, Zheng Jingmin. Research Progress on the Anti-Osteoporosis Effects of Plant Flavonoids [J]. Chinese Journal of Osteoporosis, 2021, 27(10): 1529-1533.
[6] Liu Xiaoyan, Xia Tian Shuang, Dong Zhiming, et al. Application and Prospects of Hops in the Treatment of Osteoporosis [J]. Journal of Pharmaceutical Practice, 2020, 38(6): 492-495.
[7]Aggarwal D,Upadhyay SK,Singh R,et al. Recent patents on therapeutic activities of xanthohumol:a prenylated chalconoid from hops (Humulus lupulus L.) [J]. Pharm Pat Anal,2021,10(1):37-49.
[8]Okamoto K,Nakashima T,Shinohara M,et al. Osteoimmunol- ogy:The Conceptual Framework Unifying the Immune and Skeletal Systems [J]. Physiol Rev,2017,97(4):1295-1349.
[9]Muruganandan S,Ionescu AM,Sinal CJ. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives [J]. Int J Mol Sci,2020, 21(7):2277.
-
Prev
Natural Hop Extracts Empower Innovation in Bone Health Products
-
Next
Natural Hop Extract Fuels Innovation in Wellness Product Formulations


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese



