Natural Black Rice Cyanidin 3 Glucoside: Driving Functional Food Innovation
Against the backdrop of continuously upgrading health consumption, the functional food market is embracing new opportunities. Consumers increasingly favor health products with natural ingredients, clear functional benefits, and scientific backing, driving the food industry to accelerate its transition toward a dual focus on “nutrition + function.” Natural active ingredients offering comprehensive health benefits—such as antioxidant properties and metabolic support—are gradually becoming key breakthroughs for product innovation.
Cyanidin-3-glucoside (C3G), a widely distributed anthocyanin compound with significant bioactivity, emerges as an ideal functional ingredient aligning with this trend. Abundantly present in various dark-colored plants, particularly with exceptional levels in black rice, it not only imparts a vivid purple-red hue to foods but also possesses outstanding antioxidant and physiological regulatory potential, making it the preferred ingredient for developing high-value functional foods.
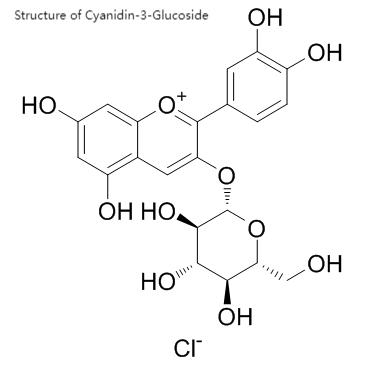
Green Spring Technology leverages advanced extraction and purification processes to introduce a high-purity, highly stable natural black rice extract C3G ingredient, offering functional food enterprises the following core advantages:
✨ High Activity & Purity: Multi-step chromatographic purification ensures consistent C3G purity exceeding 95%, guaranteeing potent efficacy and batch-to-batch consistency;
✨Natural Origin & Clean Label: Derived entirely from natural black rice, aligning with clean label trends and meeting consumer demands for “natural, safe, and transparent” products;
✨Exceptional Stability: Microencapsulation and formulation adaptation technologies effectively address anthocyanin stability challenges during processing and storage, extending product shelf life;
✨Comprehensive Application Support: Full-process technical assistance, including ingredient testing, formulation recommendations, and stability solutions to accelerate product development and innovation.

Green Spring Technology is committed to establishing black rice C3G as a star ingredient in the functional food sector, continuously providing industry partners with scientifically credible, cost-effective natural ingredient solutions to jointly advance the innovation and upgrading of next-generation functional foods. Discover Green Spring Technology's comprehensive standardized black rice extract ingredient solutions to infuse your products with stable competitiveness.
1 Breakthrough in High-Purity C3G Technology: Black Rice Extract Injects Core Momentum into Functional Food Innovation
The successful purification and quantitative analysis of high-activity cyanidin-3-glucoside (C3G) from black rice extract delivers premium raw materials and scientific backing for functional food innovation. Multiple studies and experimental results confirm black rice as a natural, high-quality C3G source with significant content and excellent extraction/purification feasibility.
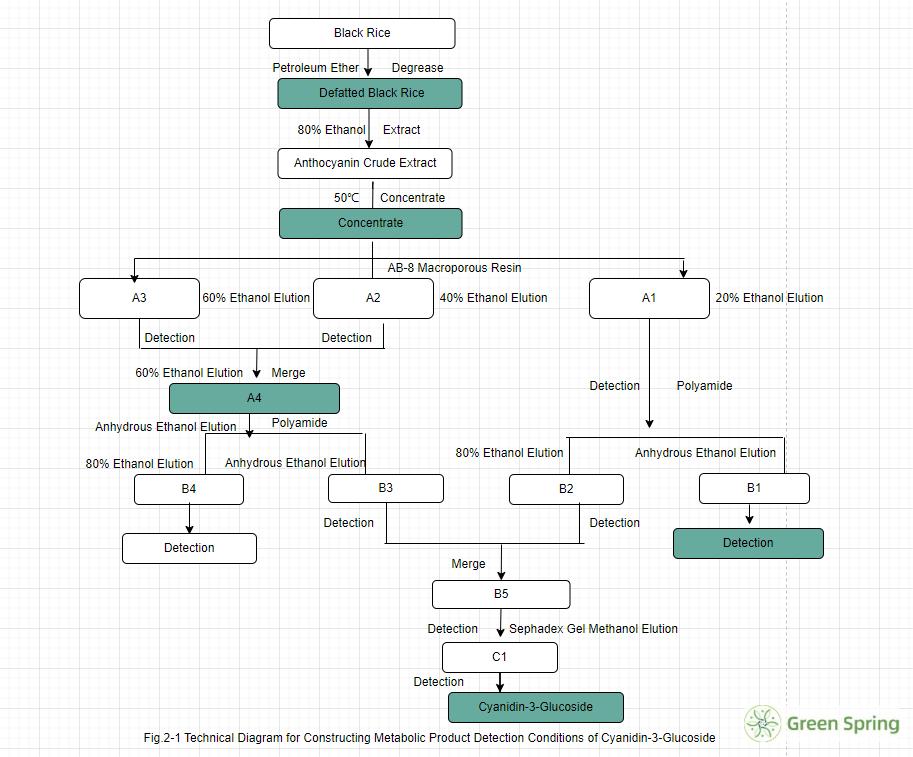
This study systematically optimized the extraction and purification process. Employing multi-step chromatography techniques—including AB-8 macroporous resin, polyamide, and dextran gel—successfully yielded high-purity C3G from black rice extract. The purity reached 95% with a yield of 0.098%, consistent with literature reports. This demonstrates the process's stability and reliability, indicating potential for large-scale application.
For qualitative and quantitative analysis, ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-MS/MS) was employed. Under multiple reaction monitoring (MRM) mode, C3G was accurately identified as the primary anthocyanin component in the black rice extract by comparing retention times with standards. A calibration curve equation was established (Y=79.027X+85.746, R²=0.9991), demonstrating excellent linearity within the range of 19.2–134.4 ng/mL. This provides a robust foundation for achieving precise quality control of C3G and standardizing the product.
Based on this purification and detection system, Green Spring Technology introduces a high-purity, high-potency C3G black rice extract, committed to delivering the following value to functional food enterprises:
⭐ Standardized C3G extract with >95% purity, ensuring consistent product efficacy;
⭐Comprehensive UPLC-MS/MS quality control methods supporting end-to-end raw material and finished product quality assurance;
⭐Stable raw material suitable for diverse food and beverage systems, enabling antioxidant and health functional innovations;
⭐Scientific validation and ingredient upgrade solutions for sports nutrition, meal replacement products, and health maintenance foods.
This research not only validates the compositional advantages and process feasibility of black rice C3G but also provides raw material assurance and theoretical support for developing high-value-added functional foods. Green Spring Technology will continue advancing C3G's innovative applications in the food sector, collaborating with industry partners to create next-generation natural, scientific, and highly effective health products.
2 Natural Black Rice C3G: An Antioxidant Solution Empowering Functional Food Innovation
Cyanidin-3-glucoside (C3G), the core active component in black rice extract, has garnered significant attention in the functional food sector in recent years due to its diverse health-supporting properties. Multiple studies indicate that C3G possesses exceptional antioxidant and anti-inflammatory potential, helping maintain normal bodily functions and providing robust scientific evidence for innovative functional food development.
In terms of antioxidant activity, C3G demonstrates superior free radical scavenging capabilities, contributing to cellular health maintenance. Research indicates C3G supports the activity of intracellular antioxidant enzyme systems while reducing levels of oxidative stress-related markers. These properties make it an ideal functional ingredient for countering daily oxidative stress.
In the realm of metabolic health, C3G demonstrates potential for metabolic regulation. Experimental studies indicate that C3G helps maintain healthy metabolic marker levels and supports the body's metabolic balance by regulating relevant biological pathways. These findings offer new perspectives for developing functional foods focused on health management.
Additionally, C3G holds positive implications for liver and kidney health. Research confirms that C3G supports normal liver and kidney function, with its beneficial effects primarily attributed to its potent antioxidant properties.
Green Spring Technology employs advanced extraction techniques to obtain high-purity, highly active C3G components from premium black rice, providing functional food enterprises with:
✨ Standardized C3G extracts ensuring consistent product quality
✨ Exceptional ingredient stability suitable for diverse food matrices
✨Scientifically validated health support properties, providing reliable backing for product innovation
✨Comprehensive traceability system, guaranteeing raw material quality and safety
As consumer demand for natural, functional foods continues to grow, C3G—a multi-functional natural active ingredient—is emerging as a key choice for functional food innovation. Green Spring Technology remains committed to advancing C3G application research, providing industry partners with premium raw materials and innovative solutions to collectively drive the development of the functional food sector.
3 Scientific Basis for Innovative Applications of Cyanidin-3-Glucoside (C3G) from Black Rice Extract in Functional Foods
In recent years, cyanidin-3-glucoside (C3G), the core active ingredient in black rice extract, has garnered significant attention for its diverse potential health-supporting properties. Although C3G levels detected directly in human blood and tissues are relatively low, research indicates that it can be metabolized into multiple active small-molecule compounds within the gastrointestinal tract and absorbed into the bloodstream. This provides a new perspective for explaining C3G's broad physiological effects.
A recent study systematically analyzed C3G's metabolic pathways and absorption in the rat gastrointestinal tract through in vivo and in vitro experiments combined with advanced UPLC-MS/MS detection technology. Using purified black rice C3G (95% purity) as experimental material, the study found that C3G metabolizes into 11 phenolic acid compounds in the gut, including protocatechuic acid, vanillic acid, and ferulic acid, primarily in the jejunum, ileum, and colon. Further blood testing revealed that all 10 metabolites except one were absorbed into the bloodstream, suggesting these metabolites may serve as the key biological basis for C3G's health-supporting effects.
This research not only elucidates the metabolic pathways and absorption mechanisms of C3G within the body but also provides crucial theoretical support for the application of black rice C3G in functional foods. Given its favorable metabolic characteristics and multifaceted biological activity, C3G demonstrates broad application potential in antioxidant, metabolic support, and overall health maintenance products.

Green Spring Technology, leveraging its leading purification processes, has successfully achieved large-scale production of high-purity, high-potency C3G, providing functional food enterprises with:
⭐Standardized C3G extracts exceeding 95% purity
⭐Core ingredients backed by comprehensive metabolic science
⭐Stable formulation solutions suitable for diverse food systems
⭐Full-chain technical support from raw materials to application
Moving forward, Green Spring Technology will deepen research and collaboration on C3G and its metabolites, driving innovative applications of black rice extract in functional foods, sports nutrition, and health diets. We empower clients to develop next-generation products backed by scientific credibility and clear functional benefits.
Contact us today at helen@greenspringbio.com or WhatsApp: +86 13649243917 to request complimentary samples, technical documentation, and application solutions. Let's collaborate to create more market-competitive functional food innovations!
Reference:
[1] Jin X., Chen M., Long Y., et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway[J]. Molecular Nutrition and Food Research, 2014,58(10):1941–1951.
[2] Xu Z., Howard L. R. Analysis of antioxidant-rich phytochemicals[M]. New Jersey, Wiley-Blackwell,2012.
[3] Jr T. A., Reese R. N., Wyzgoski F. J., et al. Cyanidin-3-rutinoside and cyaniding-3-xylosylrutinoside as primary phenolic antioxidants in black raspberry[J]. Journal of Agricultural and Food Chemistry, 2008,56(6): 1880–1888.
[4] Sousa A., Araújo P., Azevedo J., et al. Antioxidant and antiproliferative properties of 3-oxyanthocyanidins[J]. Food Chemistry, 2016, 192: 142–148.
[5] Kumar S., Gautam S., Sharma A. Identification of antimutagenic properties of anthocyanins and other polyphenols from rose ( Rosa centifolia ) petals and tea[J]. Journal of Food Science, 2013, 78(6): 948–954.
[6] Bao Rui, Chen Di, Chen Fang the Effect of Vc on Microwave Degradation of Cyanidin-3-Glucoside in Blueberry Extract [j] Preservation and Processing, 2014, 14 (6): 34-39
[7] Liu Changjiao, Xiong Xiangwei, Zheng Xia, et al HPLC method for the determination of cyanidin-3-glucoside in black rice [J] China Food Additives, 2017, (9): 210-215
[8] Wang Jing, Zhou Youxiang, Yan Wei, Et Al Identification and Content Analysis of Cyanidin-3-Glucoside in Black Rice [j] Hubei Agricultural Science, 2014, 53 (23): 5836-5838
-
Prev
Supercritical Chamomile Essential Oil for High-Performance Skincare Formula
-
Next
Black Rice Extract Solutions by Green Spring Technology


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




