How to Immobilize Papain Powder?
Papain [EC 3.4.22.2] is an important biochemical product with widespread applications in the food and pharmaceutical industries, as well as in feed, textiles, and leather processing [1]. Due to the high cost of papain and its inability to be reused, researchers have been exploring the preparation of immobilized papain. This research began in the early 1960s and has since been extensively documented in the literature [2]. The main methods for enzyme immobilization include embedding, adsorption, covalent bonding, and cross-linking, each with its own advantages and disadvantages [3]. This paper summarizes recent research and application progress on immobilized papain, compares the enzymatic characteristics such as enzyme activity, stability, and recovery rate of immobilized papain prepared using various immobilization methods in recent years, and provides suggestions.
1 Embedding Method
Embedding method: The method of embedding enzymes or enzyme-containing bacterial cells in a porous carrier to fixate enzymes is called the embedding method. Gel embedding is a method of embedding enzymes in the microporous structure of various gels to produce immobilized enzymes of a specific shape. The most commonly used gels include agar, agarose, calcium alginate, carrageenan, and polyacrylamide. Microcapsule embedding involves encapsulating the enzyme within a semipermeable polymer membrane to produce microcapsule-immobilized enzymes.
1.1 Sodium alginate-chitosan immobilized papain (IPSAC)
Alginic acid is extracted from brown algae and is a polysaccharide composed of glucuronic acid linked by β-1,4 bonds. It is an ideal carrier for enzyme encapsulation, with a simple immobilization method, mild conditions, and operations that can be conducted at room temperature, resulting in minimal enzyme inactivation. However, calcium alginate gels are unstable in solutions containing polyvalent anions (phosphates, citrates, lactates, etc.) and high concentrations of electrolytes (K+, Na+), with Ca2+ ions easily detaching, causing the gel to soften or even dissolve. He et al. [4] studied the immobilization of papain using sodium alginate-chitosan in two aspects: first, they used Tris-hydrochloric acid buffer solution during gel preparation to reduce Ca²⁺ ion detachment and maintain gel stability; second, they first added chitosan powder to enhance gel hardness. The positive charges (-NH₂) on chitosan interact with the carboxyl groups of alginate, stabilizing the gel, making it suitable for column loading. The enzyme activity of the immobilized enzyme was the same as that of the enzyme without chitin powder.
In He et al.'s [4] study, the optimal pH value for PA and IPSAC was 7.2, and the activity remained stable at temperatures below 70°C. The spatial structure of the embedded PA did not change, so the optimal pH value and thermal stability remained essentially unchanged. The half-life of IPSAC is 59 days.
Sodium alginate-chitosan immobilized papain (IPSAC) exhibits good thermal stability, a long half-life, and high gel hardness, making it suitable for column loading and convenient for automated production in industrial settings.
1.2 Chitosan capsule method for immobilizing papain
Chitosan is a biopolymer obtained by deacetylation of chitin, also known as deacetylated chitin. As an enzyme carrier, chitosan has advantages such as chemical stability and good thermal stability, making it an excellent carrier for immobilized enzymes.
Huang Zeyuan [5] used chitosan and sodium carboxymethyl cellulose to form capsules for the immobilization of papain, determining the optimal conditions for enzyme immobilization as follows: enzyme loading of 500 mg/10 mL, chitosan concentration of 0.2%, pH of 7.5, and temperature of 50°C. The activity recovery rate of the immobilized papain reached as high as 69.8%.
2. Adsorption method
Adsorption method: The method of immobilizing enzymes or enzyme-containing bacterial cells by adsorbing them onto the surface of a solid adsorbent is called the adsorption method. Common adsorbents include activated carbon, aluminum oxide, diatomaceous earth, porous ceramics, porous glass, silica gel, and carboxyphosphate. The adsorption method for preparing immobilized enzymes is simple to operate, has mild conditions, does not cause enzyme denaturation or inactivation, uses inexpensive and readily available carriers, and can be reused. However, due to the weak physical adsorption forces, the enzyme-carrier complex is unstable and prone to detachment, limiting its practical application in industrial production. Research in this area is also relatively scarce.
2.1 Freeze-vacuum adsorption method for immobilizing papain in mesoporous materials
There are various methods for assembling functional materials in the pores of mesoporous molecular sieves, including gas-phase adsorption and impregnation. Compared to conventional adsorption methods, the freeze-vacuum adsorption method is a new approach with the following advantages: first, it provides a strong driving force, resulting in higher protein adsorption; second, it maintains enzyme activity at low temperatures.
Gao Bo et al. [6] used mesoporous material SBA-15 as a carrier to prepare immobilized papain. Comparing the characteristics of immobilized enzymes and solution enzymes, they found that the optimal temperature for papain increased from 60°C to 70°C after immobilization. The optimal pH value for the enzyme after immobilization was 7.0, with no change in the optimal pH value, which may be attributed to the neutral surface charge properties of the mesoporous molecular sieve SBA-15. A certain amount of immobilized enzyme and solution enzyme were taken, incubated at 30–90°C for 30 minutes, rapidly cooled to room temperature, and then enzyme activity was measured at 37°C.

After papain was assembled into molecular sieves, its thermal stability was significantly enhanced. Even after being incubated at 90°C for 30 minutes, it retained 70% of its activity, while the solution enzyme had already lost its activity at 90°C. This indicates that assembling the enzyme into molecular sieves can enhance thermal stability. Since high temperatures not only increase reaction rates, reduce bacterial contamination, but also enhance substrate solubility, thereby improving yield, the immobilized enzymes prepared using mesoporous material SBA-15 as a carrier exhibit excellent thermal stability, making them more suitable for industrial applications.
Experimental results indicate that under appropriate vacuum conditions, the freeze-vacuum adsorption method can be used to prepare immobilized enzymes with high assembly efficiency, and the enzyme activity remains good with high stability. Therefore, freeze-vacuum adsorption can serve as a new method for immobilizing enzyme proteins.
3 Covalent Coupling Method
Covalent coupling method: The method of preparing immobilized enzymes by covalently bonding non-essential groups outside the enzyme active center with groups on the solid-phase carrier is called the covalent coupling method, also known as the covalent bonding method. The covalent bonding method is one of the most actively researched immobilization methods currently. Its advantages include strong bonding between the enzyme and the carrier, resistance to detachment, and the ability to be used continuously for extended periods.
However, it also has the following drawbacks: (1) During the immobilization process, direct (rigid) collisions occur between enzyme molecules and carrier molecules, which can alter the enzyme's conformation and cause inactivation; (2) Carriers are generally hydrophobic substances. When enzymes are directly immobilized on hydrophobic carriers, the enzyme's microenvironment is altered, leading to conformational changes, folding, or denaturation of the enzyme protein; inactivation; (3) The traditional covalent coupling method results in immobilized enzymes with low freedom of movement, and there is significant steric hindrance between the enzyme molecules and substrates, which is unfavorable for maintaining the homogeneous catalytic activity of the free enzyme state. Therefore, this method is referred to as “rigid immobilization.”
Common carriers for covalent coupling include cellulose, dextran, agarose, and chitin. Here, we focus on several newer covalent coupling methods for immobilizing papain.
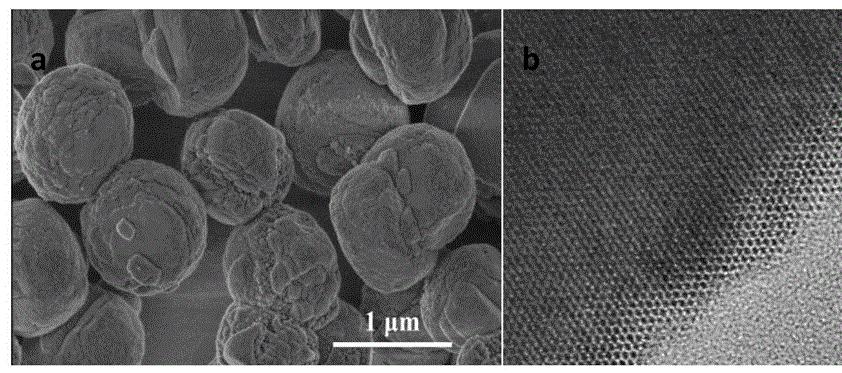
3.1 Flexible Immobilization of Papain Using Bis-Aldose Starch
To address the issues with covalent coupling methods, researchers proposed attaching short chains (arm molecules) to the carrier surface to achieve “arm-based immobilization” of enzymes. However, since these arm chains are small-molecular-weight short chains and some are hydrophobic carbon chains, enzyme molecules still experience significant impact forces during immobilization, leading to conformational changes and inactivation. Wang Haiping, Wei Rongqing, et al. [7–8] proposed the “flexible immobilized enzyme” model, which involves attaching hydrophilic molecular chains of sufficient length to the immobilization carrier, referred to as “flexible chains,” and then binding the enzyme to the flexible chains. The following steps were used to achieve the flexible immobilization of papain, as shown in Figure 1.
Using the enzyme flexible immobilization model, a flexible carrier (Chitosan-DAS50) was prepared using chitosan and bis-aldehyde starch, and papain was flexibly immobilized. The activity recovery rate of the immobilized enzyme reached 72%, which is three times that of the chitosan-glutaraldehyde (Chitosan-GA) arm carrier. The flexibly immobilized papain retained half of its activity after 7–8 batches and exhibited significantly higher storage stability than free enzyme.
The results indicate that the “flexible immobilization” method not only reduces enzyme inactivation during the immobilization process but also ensures that the immobilized enzyme retains higher homogeneous catalytic activity compared to free enzyme.
3.2 Immobilization of Papain on Magnetic Polymer Microspheres
Magnetic polymer microspheres are ultra-fine powders containing magnetic metals or metal oxides (such as iron, cobalt, nickel, and their oxides) and exhibiting magnetic responsiveness. They are a novel functional polymer material developed over the past two decades. Through copolymerization, surface modification, and other chemical reactions, various reactive functional groups (such as hydroxyl, carboxyl, aldehyde groups, and amino groups) through chemical reactions such as copolymerization and surface modification. These functional groups can form covalent bonds with bioactive substances such as enzymes and antibodies. Under the influence of an external magnetic field, these microspheres can undergo rapid movement or separation. Therefore, magnetic polymer microspheres, as a new type of functional polymer material, have broad application prospects in fields such as biomedicine, bioengineering, and enzyme immobilization.
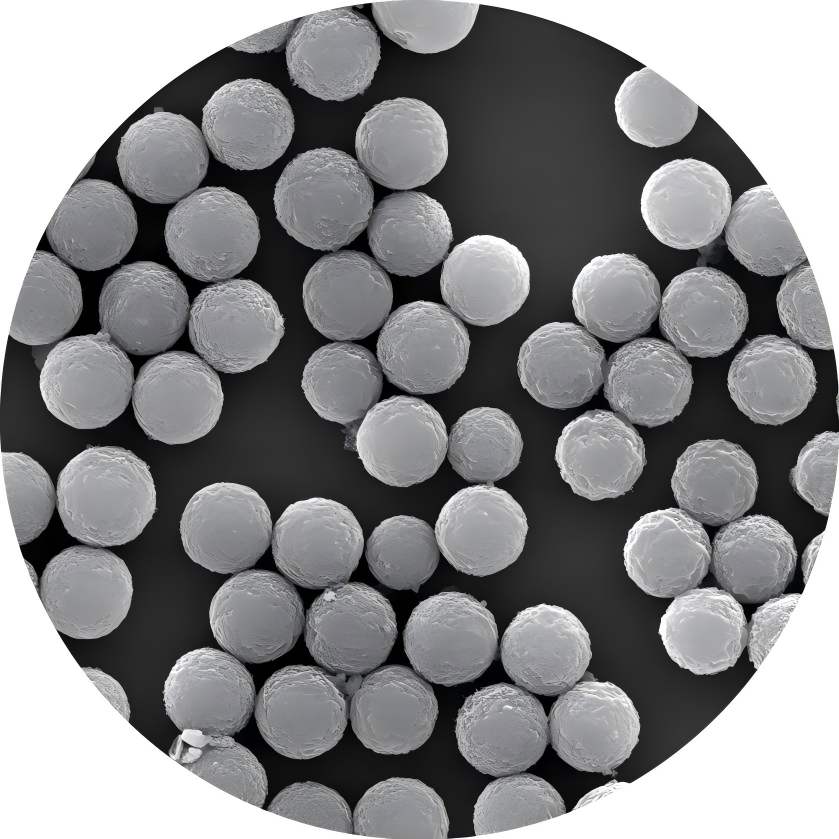
Zeng Lixi et al. [9] synthesized a highly hydrophilic, uniformly sized, well-dispersed magnetic polymer porous composite microsphere with a surface rich in functional epoxy groups, which was used for the immobilization of papain. Using casein as the substrate, the enzymatic properties and kinetic parameters of immobilized enzymes were studied. The results showed that the optimal reaction temperature for immobilized enzymes was 80°C. After incubation at 90°C for 40 minutes, the immobilized enzymes retained 65% of their original enzyme activity, while the solution enzymes only retained 6.6% of their original enzyme activity. The optimal reaction pH was 7.5. When the pH ranged from 6.0 to 8.5, the immobilized enzyme consistently exhibited higher activity than the free enzyme, with an enzyme activity recovery rate of 55.2%. The immobilized enzyme demonstrated better acid-base tolerance and significantly improved thermal stability, pH stability, and operational stability compared to the free enzyme.
Magnetic polymer porous composite microspheres immobilized papain, this carrier possesses excellent hydrophilicity, biocompatibility, large surface area, and a high content of functional epoxy groups, enabling the preparation of highly active and performant immobilized enzymes. Utilizing the magnetic responsiveness of the carrier, the enzyme can be easily separated from the reaction system under an external magnetic field, making this a trend in the use of catalysts for biochemical reactions.
3.3 Metal chelation carrier-directed immobilization of papain
Metal chelation affinity chromatography (IMAC) utilizes the coordination bonding between metal chelating ligands and electron-donating amino acids on the protein surface, such as the imidazole group of histidine and the thiol group of cysteine (with histidine being the most important), to achieve the separation and purification of proteins. Metal chelation carriers have minimal impact on the conformation of proteins immobilized on their surfaces and can be reused. Applying this technique to immobilized enzymes holds promise for overcoming some of the limitations of currently used immobilization carriers and methods.
Liu Linlin et al. [10] used magnetic metal chelating agarose microspheres as carriers and exploited the interaction between metal chelating ligands (IDA-Cu²⁺) and electron-donating amino acids on the protein surface to directionally immobilize papain. The optimal immobilization conditions were Cu²⁺ 1.5 × 10^(−2) mol/g carrier, immobilization time 4 h, immobilization pH 7.0, and enzyme loading 30 mg/g carrier. The optimal reaction temperature for the immobilized enzyme is 70°C, and the optimal reaction pH is 8.0. The thermal stability of the immobilized enzyme is significantly higher than that of the solution enzyme. The enzyme activity recovery of the immobilized enzyme is 68.4%, and it exhibits good operational stability. After five repeated uses of the carrier, the enzyme activity of the immobilized enzyme is 79.71% of that of the initially immobilized enzyme.

The results indicate that when using metal chelating carriers for the directed immobilization of papain, the immobilization reaction conditions are mild, with minimal impact on the enzyme's higher-order structure, and high enzyme activity recovery; the thermal stability of the immobilized enzyme is significantly improved; the carrier has a high enzyme loading capacity; the regeneration of the carrier and the immobilization of the enzyme are simple to operate; the immobilized enzyme and carrier can be reused multiple times, and the production cost of the immobilized enzyme is low. Therefore, this technology has broad application prospects in industry.
3.4 Soluble Immobilized Papain
Immobilized enzymes prepared by traditional methods have advantages such as good stability and convenient recovery, but their application is limited when dealing with insoluble, high-viscosity substrate solutions, resulting in low catalytic efficiency. The low catalytic efficiency of enzymes is attributed to the enzyme-catalyzed reaction occurring in a non-homogeneous reaction system between the solid and liquid phases, resulting in significantly lower enzyme-substrate contact probabilities compared to homogeneous free enzyme reaction systems. Therefore, the focus of improving the catalytic efficiency of immobilized enzymes should be on optimizing the catalytic reaction system and preparing immobilized enzymes capable of forming a homogeneous catalytic reaction system.
Li Lingling et al. [11] conjugated papain (PP) with carboxymethyl cellulose acetate succinate (As-L) to prepare water-soluble immobilized PP. The optimal pH values for free and water-soluble immobilized PP were determined to be 6.0 and 5.0, respectively, with optimal reaction temperatures of 60°C and 70°C, and Km values of 2.53 and 3.07 mg·mL, respectively. The soluble immobilized PP retained 62% of its activity after 12 hours of incubation at 60°C, 62% of its activity remained; at 4°C, after 30 days, the activity retained over 90%; at a pH of 6.0, the stability was best, and it remained relatively stable within a pH range of 4.0–7.0 (relative activity >80%). Soluble immobilized PP can completely dissolve in solutions with a pH greater than 5.5, with a narrower optimal pH range, increased optimal temperature, and higher Km value; thermal and acid-base stability are significantly enhanced.
Soluble immobilized papain combines the high catalytic activity of free papain with the high stability and ease of recovery of insoluble immobilized enzymes, offering broad application prospects in industrial applications.
4 Cross-linking method
Cross-linking method: The cross-linking method involves using bifunctional reagents to induce cross-linking between enzyme molecules or between enzyme molecules and solid-phase carriers to produce immobilized enzymes. Commonly used bifunctional reagents include glutaraldehyde, hexamethylenetetramine, maleic anhydride, and bis(2-nitro-5-nitrophenyl)amine. Among these, glutaraldehyde is the most widely used. Enzymes immobilized by the cross-linking method exhibit strong binding and can be used for extended periods. However, due to the vigorous nature of the cross-linking reaction, multiple groups of the enzyme molecules are cross-linked, resulting in significant loss of enzyme activity. In practical applications, this method is often combined with other immobilization methods, such as gel encapsulation followed by cross-linking. This technique, which employs two or more methods for immobilization, is referred to as dual or multiple immobilization. Using this method, immobilized enzymes with high enzyme activity and good mechanical strength can be prepared.
Cross-linking is the most commonly used method for immobilizing papain. Research in this area began early in China [12–13], so we will only list some of the latest research applications of cross-linking methods for immobilizing papain on new carrier materials.
4.1 Silk-immobilized papain
Chen Fangyan et al. [14] used activated silk fibroin as a carrier and employed a covalent cross-linking method to immobilize papain, investigating the enzymatic properties of silk fibroin-immobilized papain. The results indicated that, due to silk fibroin being a hydrophilic polymer, the immobilized papain prepared using silk fibroin as a carrier exhibited significantly enhanced affinity for the substrate. the apparent Michaelis constant Km app[casein] of the immobilized enzyme was 0.092%, which was 0.46 times that of the solution enzyme (Figure 2); the optimal pH was 7.5; at pH values between 6.5 and 8.0, the enzyme activity remains stable within a temperature range of 4.0–55°C; the operational half-life of the solution enzyme is 38 days, while that of the immobilized enzyme is 54 days. The operational half-life of the immobilized enzyme is significantly longer than that of the solution enzyme.
4.2 Immobilized papain using ovalbumin as a carrier
Huang Yibing et al. [15] used denatured ovalbumin directly as a carrier, combined with traditional cross-linking immobilization methods, and successfully immobilized papain using glutaraldehyde as a cross-linking agent, simplifying the operational steps. Studies have shown that immobilized papain using ovalbumin as a carrier exhibits optimal reaction temperatures of 60°C for the native enzyme and 90°C for the immobilized enzyme under the same reaction conditions.
The optimal reaction temperature of the immobilized enzyme was increased. The optimal pH value of the immobilized papain also shifted toward alkalinity to 8.0, and the acid stability of papain immobilized using ovalbumin powder as a carrier was significantly improved. After being treated in a boiling water bath at 100°C for 5 hours, the immobilized papain retained 54.6% of its activity, while the solution enzyme completely lost its enzymatic activity after 2.5 hours of treatment. The experimental results indicate that immobilized papain exhibits enhanced stability under environmental conditions. Stability is a critical factor determining the practical applicability of immobilized enzymes. In most cases, enzyme stability increases after immobilization, which is highly advantageous.
4.3 Chitosan-immobilized papain
Chitosan-immobilized papain is currently a relatively mature method for immobilizing papain [16–18]. The main method involves using chitosan as a carrier and glutaraldehyde as a cross-linking agent to prepare immobilized papain [18]. Using chitosan as a carrier, the method for preparing immobilized papain is simple, with mild immobilization conditions, and the resulting immobilized enzyme exhibits significantly improved heat resistance and thermal stability, making it a commonly used method for immobilizing papain.
5 Summary and Outlook
There are numerous methods for preparing immobilized papain. From these extensive studies, it can be observed that after immobilization, enzymes undergo certain changes in their characteristics due to the influence of carriers and other factors. Therefore, during the application of immobilized enzymes, it is essential to understand the differences in properties between immobilized enzymes and free enzymes, as this knowledge is crucial for optimizing their application. By adjusting the process accordingly, the enzymes can be operated under optimal conditions to catalyze reactions efficiently. Due to differences in experimental designs among researchers, it is challenging to evaluate the advantages and disadvantages of various immobilization methods. Immobilized papain prepared using different methods has different application ranges, and the appropriate method should be selected based on actual needs to better serve production.
After being immobilized using various methods, papain exhibits enhanced stability, increased optimal temperature, broader pH tolerance range, improved enzyme activity, and extended half-life, indicating that the immobilization method is appropriate. Further optimization should be pursued to achieve better results. Based on the existing research findings, further improving the activity and substrate affinity of immobilized enzymes is a key focus for future research.
Summarizing the recent progress in the study of immobilized papain, it can be seen that with the continuous advancement of technology, the catalytic activity and stability of immobilized papain are satisfactory. Enzyme immobilization technology enables the repeated use of enzymes and the effective separation of raw materials, products, and enzymes, thereby simplifying separation processes and equipment, shortening production cycles, and reducing production costs. Immobilized enzymes will become an important application form of enzymes in industry. The increasing maturity of enzyme immobilization technology will enable papain to play an even greater role in food, beverages, pharmaceuticals, chemical reagents, feed, textiles, and cosmetics.
References
[1] Jone J G. Refined papain [J]. Process Biochemistry, 1974, 9(6):21–24.
[2] Xiong Hua. Research progress on the application of papain [J]. Preservation and Processing, 2006(1):7–8.
[3] Yao X L, Ku H S, Song W J. Study on enzymatic characteristics of immobilizing trypsin with different carriers [J]. Biotechnology, 2007, 17(3): 71–73.
[4] He Ping, Huang Zhuolie, Li Chuny, et al. Immobilization and properties of papain [J]. Journal of Tropical and Subtropical Botany, 2008, 16(4): 334–338.
[5] Huang Zeyuan. Study on the immobilization of papain using chitosan capsules [J]. Food Science and Technology, 2002(12): 10–12.
[6] Gao Bo, Zhu Guangshan, Fu Xueqi, et al. Immobilization of papain in mesoporous materials using freeze-vacuum adsorption [J]. Journal of Jilin University, 2005, 43(6): 66–71.
[7] Wang Haiping, Wei Rongqing, Shen Bin, et al. Study on the Flexible Immobilization of Papain Using Bis-Aldose Starch [J]. Bioprocess Engineering, 2004, 2(1): 25–29.
[8] Wei Rongqing, Shen Bin, Liu Xiaoning, et al. Flexible Immobilization of Papain Using Chitosan Carrier [J]. Journal of Process Engineering, 2005, 5(2): 183–187.
[9] Zeng Lixi. Study on the Activity and Stability of Papain Immobilized on Novel Magnetic Polymer Porous Microspheres [J]. Journal of Natural Sciences, Hunan Normal University, 2007, 30(1): 101–106.
[10] Liu Linlin, Zeng Lixi, Liu Ting, et al. Study on the directed immobilization of papain on metal chelate carriers [J]. Journal of Bioengineering, 2005, 20(5): 26–31.
[11] Li Lingling, Zhang Tao, Yu Rong, et al. Preparation and properties of soluble immobilized papain [J]. Journal of West China Pharmacy, 2007, 22(2): 77–79.
[12] Tao Guoliang, Li Yanfeng. Study on the Immobilization of Papain on Ammonia-Treated Macroporous Spherical Polyvinyl Chloride [J]. Applied Chemistry, 1993, 10(2): 9–12.
[13] Tan Huiying, Chen Xuelin. Immobilization and Infrared Activation of Papain on Microbeads [J]. Biotechnology, 1993, 3(5): 17–20.
[14] Chen Fangyan, Ji Pingxiong. Study on the Characteristics of Silk-Fixed Papain [J]. Journal of South China Agricultural University, 2005, 26(4): 81–83.
[15] Huang Yibing, Wu Xiaoxia, Wu Shengnan, et al. Study on the Immobilization of Papain Using Ovalbumin as a Carrier [J]. Journal of Jilin University, 2004, 42(4): 15–20.
[16] Huang Jianshao, Zhang Hong. Chitosan-Immobilized Papain [J]. Journal of Changde Normal University (Natural Science Edition), 2002, 14(1): 36–39.
[17] Yuan Chuntao, Jiang Xianming. Study on Chitosan-g-Acrylonitrile-Immobilized Papain [J]. Applied Chemistry, 2002, 19(9): 28–31.
[18] Liao Qiu Hua. Study on the Immobilization of Papain by Microcrystalline Chitosan [J]. Journal of Guangzhou Military Medical College, 1998, 21(1): 30–32.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese