What Are the Uses of Papain Powder in the Food Field?
Papain, a protease enzyme derived from the fresh latex of unripe papaya (Carica papaya) fruit, is an endopeptidase containing sulfhydryl (-SH) peptide chains. It exhibits protease and esterase activities with broad specificity, effectively hydrolyzing animal and plant proteins, peptides, esters, and amides. It also has synthetic functions, capable of synthesizing protein hydrolysates into protein-like substances. Industrially used papain is generally an unpurified multi-enzyme system. It is known that papain obtained by drying papaya latex contains at least four major enzyme types: papain, chymopapain, papain proteinase Ω (papaya proteinase Ω), and chymopapain M (chymopapain M) [1], among which chymopapain has the highest content, accounting for 45% of soluble proteins.
Papain is highly soluble in water and glycerol, forming colorless or pale yellow solutions, sometimes appearing milky white; it is virtually insoluble in organic solvents. Its optimal pH is 5.7 (generally effective between 3 and 9.5), and it remains active under neutral or slightly acidic conditions; the optimal temperature is 55–60°C (generally effective between 10 and 85°C), with strong heat resistance, remaining active even at 90°C; it is inhibited by oxidizing agents and activated by reducing agents.
1 Extraction process of papain
1.1 Conventional extraction methods in the past
The most primitive method for extracting papain was the drying method, which involved adding a protective agent to papaya pulp, centrifuging the pulp, collecting the supernatant, and drying it in a forced-air drying oven at 55–60°C. The dried material was then ground to obtain crude enzyme products. Yi et al. [2] obtained an enzyme yield of 23.1% using this method, but it was unfavorable for enzyme activity retention, with enzyme activity of only 0.16 × 10⁵ U/g, and the product purity was relatively low.
The extraction of papain has also been widely studied using the tannin precipitation method. Yi et al. [2] extracted papain using this method. First, the papaya juice was centrifuged, and a solution of dissolved tannin was slowly added to the supernatant while stirring continuously until the tannin concentration in the solution reached a certain level. The solution was then allowed to stand to precipitate the tannin-enzyme complex, the pH was adjusted, and the precipitate was vacuum-dried to obtain the enzyme product. The results showed that this method yielded a low enzyme yield of only 7.3%, but the enzyme activity was relatively high at 3.53 × 10⁵ U/g. However, this method has issues such as environmental pollution.

1.2 Currently Commonly Used Extraction Methods
Currently, ultrafiltration, flocculation, and salt precipitation are commonly used methods for separating and extracting papain.
1.2.1 Ultrafiltration method for extracting papain
Tan Jing et al. [3] used ultrafiltration technology to separate papain. First, hollow fiber ultrafiltration membranes were cleaned and treated, then ultrafiltration was performed under controlled pressure and flow rate. Papain is a macromolecular substance that is retained during ultrafiltration, while water and small molecular impurities pass through the membrane, thereby achieving separation and purification. Experimental results showed that the activity of papain after ultrafiltration was 1.22 times that before ultrafiltration, and the protein content was 2.8 times that before ultrafiltration, indicating that the purity of papain increased after ultrafiltration. However, since papain is a macromolecular substance, it tends to accumulate on the membrane surface during ultrafiltration, causing concentration polarization and gradually reducing the ultrafiltration rate, thereby affecting enzyme yield.
1.2.2 Salt precipitation method for extracting papain
Wang Libin et al. [4] previously used ammonium sulfate precipitation to separate papain. A solution of ammonium sulfate at a certain concentration was added to the crude enzyme solution for salting-out, followed by adjusting the pH, centrifuging to collect the precipitate, drying, and obtaining the enzyme product. Experimental results showed that the papain prepared by this method had relatively high purity, with an enzyme specific activity of 1184 U/mg, making it suitable as a cosmetic ingredient. However, this method uses a large amount of salt, increasing the ash content in the enzyme and simultaneously reducing the activity of papain [5]. Therefore, the salt precipitation method is not an ideal method for extracting papain.
1.2.3 Flocculation method for the separation and purification of papain
The flocculation method involves adding a certain compound to the papain latex water extract, which forms insoluble complexes with papain, causing the enzyme to precipitate and separate from the solution. He Jiqin [6] et al. studied the purification of papain using flocculation technology. Experimental results showed that under appropriate conditions, flocculation treatment could achieve a yield of 95–97% for papain in this step. However, this method has low specificity, resulting in papain with low purity and high impurity content.
1.3 New Technologies for the Extraction of Papain
1.3.1 Ultrasonic Extraction of Papain
Ultrasonic extraction, as a novel extraction technique, has been widely applied in the extraction of active components from natural plants. The cavitation effect generated by ultrasonic waves [7] enhances the solvent's penetration into cell walls, intensifies mass transfer between the cell interior and exterior, and disrupts cell walls, thereby facilitating the release of intracellular components and enhancing the extraction process [8]. The microjet effect formed by ultrasonic waves is also an important factor in improving extraction efficiency.
Xiao Guiping [9] previously used ultrasonic extraction to extract papain from papaya. Fresh papaya was selected, washed, cut into pieces, and crushed. The fruit pulp was then blended into a paste. The pulp was subjected to ultrasonic treatment under specific conditions, followed by centrifugation, purification, and filtration. The supernatant was then concentrated via ultrafiltration to collect the enzyme solution sample. The results showed that at an ultrasonic power of 300 W, an ultrasonic treatment time of 200 s, and a fruit pulp mass fraction of 30%, the enzyme activity was 1.71 times higher than that of the untreated sample.
The crude enzyme solution obtained using the ultrasonic extraction process has a low enzyme content and contains a significant amount of impurities. After preliminary purification and concentration via membrane separation, further purification is required. This warrants further investigation to enhance enzyme activity and purity.
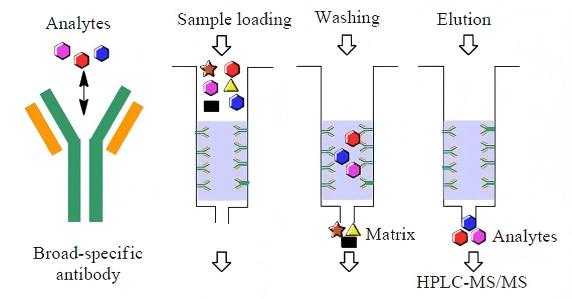
1.3.2 Affinity Membrane Chromatography for Papain Extraction
Affinity membrane chromatography is a new technology for the separation and purification of biomacromolecules developed in the late 1980s. It combines membrane separation with affinity separation, thereby incorporating the characteristics of both membrane separation and affinity separation. Nie Huali et al. [10] used nylon membrane as the substrate, modified its surface with chitosan to reduce non-specific adsorption, and coupled the dye ligand Cibacron Blue F3GA to obtain a new type of affinity membrane chromatography material, which was then used to separate papain. The results showed that the affinity membrane exhibited excellent chromatographic performance, with a high adsorption capacity for papain (235.3 mg/g). Using this affinity membrane to separate and purify papain from papaya powder, the purification factor reached 46.5-fold.
Compared with traditional membrane separation and affinity chromatography, affinity membrane chromatography not only has advantages such as high purification factor, low pressure drop, short analysis time, and low probability of denaturation of biomolecules during separation, but also allows for faster feed rates. Additionally, it is more easily scalable for large-scale purification and separation compared to column affinity chromatography [11].
1.3.3 Extraction of papain using dual-phase extraction
Double-phase extraction is a method that utilizes the differences in distribution coefficients between two immiscible phases to extract substances. Sarote N et al. [12] used the double-phase method to extract papain, and the results showed that the double-phase system composed of 8% polyethylene glycol and 15% ammonium sulfate yielded the best extraction efficiency for papain, with an enzyme specific activity of 1,659 U/mg and a yield of 86.2%.
Biphasic water extraction offers advantages such as high yield of the target product, ease of continuous operation, no residual organic solvents, and economical separation processes. It should have a place in the preparation of papain; however, no such reports have been published in China to date. With the continuous development of papain applications, the purity requirements for papain are also increasing. Therefore, there is an urgent need for more specific methods for preparing papain. Developing dual-phase extraction technology as an effective method for the separation and extraction of high-purity papain is of great significance.
In summary, the extraction methods for papain are generally not typical and typically involve a combination of methods such as salt precipitation, filtration, centrifugation, concentration, crystallization, and drying. The extraction process can be optimized based on the physicochemical properties of the enzyme to maximize resource utilization and achieve the highest possible economic benefits.
2 Immobilization of papain
Due to the high cost and non-reusability of papain, researchers have explored the preparation of immobilized papain. Immobilized papain can avoid inactivation in acidic, alkaline, and organic media, enabling repeated use of the enzyme and reducing production costs. Common immobilization methods include adsorption, carrier cross-linking, and encapsulation.
2.1 Adsorption Method
The method of adsorbing enzymes or enzyme-containing bacterial cells onto the surface of a solid adsorbent to immobilize the enzymes is called adsorption. Commonly used adsorbents include activated carbon, aluminum oxide, diatomaceous earth, porous ceramics, porous glass, silica gel, and carboxylapatite.
Fang Huan et al. [13] used porous ceramic membranes treated with phosphate-buffered solution as carriers for immobilized enzymes and employed physical adsorption to immobilize papain. The results showed that when the solution enzyme concentration was 1.0–2.0 mg/mL, pH was 7.0, and the enzyme was immobilized for 2 hours, the highest enzyme activity reached 111.1 U/g, with an activity recovery of 57.9% and a half-life of 54 days.
2.2 Carrier cross-linking method
The cross-linking method involves using bifunctional reagents to induce cross-linking between enzyme molecules or between enzyme molecules and solid-phase carriers to prepare immobilized enzymes. Commonly used bifunctional reagents include glutaraldehyde and hexamethylenetetramine.
Gao Mingxia et al. [14] used chitosan as the carrier and glutaraldehyde as the cross-linking agent to immobilize papain. The results indicated that the optimal conditions for chitosan-immobilized papain were: chitosan concentration of 2.5%, enzyme loading of 0.3 g/g carrier, reaction time of 6 hours, temperature of 15°C, pH 7.5, and enzyme activity recovery rate of 38.98%. The immobilized enzyme retained over 50% of its activity after five reuses.
2.3 Embedding method
The method of embedding enzymes or enzyme-containing bacterial cells in a porous carrier to immobilize enzymes is called the embedding method.
Jin Feng [15] used a microporous starch-sodium alginate embedding method to immobilize papain, and the results showed that the optimal conditions for preparing immobilized papain were microporous starch concentration of 4%, sodium alginate concentration of 3%, and CaCl₂ concentration of 5.5%. At this point, the optimal pH value for the immobilized papain was 5.7, and the optimal temperature was 72°C. Its thermal stability, operational stability, and mechanical strength were all improved to varying degrees, with low operational costs, making it suitable for industrial application.
2.4 Other New Methods
In recent years, researchers have continuously developed new immobilization carriers and methods, achieving promising results. Zou Zhechang et al. [16] immobilized papain using silica mesoporous foam material as a carrier. The results showed that the optimal reaction temperature of immobilized papain was 10°C higher than that of free enzyme, the optimal pH shifted 0.5 units toward the alkaline direction, and enzyme activity retained 65.1%. Lin Yin et al. [17] chemically modified high-boiling-point alcohol lignin and enzymatically hydrolyzed lignin to enhance their hydrophilicity, synthesizing novel high-polymer high-boiling-point alcohol lignin phenol (HBS lignin phenol) and lignin amino phenol derivatives, and investigated the immobilization of papain using these materials. The results showed that the activity recovery rate of papain when adsorbed by enzymatically modified lignin amino phenol and HBS lignin phenol reached over 50%, indicating its potential as an excellent carrier for immobilized enzymes.
3 Current Applications of Papain in the Food Industry
3.1 As a Meat Tenderizer
With the improvement of living standards, after solving the problem of food security, people have gradually developed higher demands for the quality and texture of meat. The meat of aged livestock and poultry, when cooked, has a coarse and hard texture. However, when tenderizing powder is added, the meat becomes tender and soft.
One of the main components of meat tenderizer is papain, which is a cysteine protease capable of degrading proteins in muscle fibers and connective tissue [18]. It breaks down myosin and collagen into smaller peptides or even amino acids, causing the muscle fibers and tendon fibers to rupture, making the meat tender, smooth, and crispy. This simplifies the protein structure, making it easier to digest and absorb. On the other hand, tenderizers work most effectively during the steaming or boiling process when temperatures are high. Papain is highly heat-stable, remaining active even at 90°C, making it ideal for producing tenderizing powder.
Lei Changgui et al. [19] used fresh beef as raw material to study the effects of papain on the muscle fiber fragmentation index (MFI) and shear force of beef. The results indicated that a papain solution at a concentration of 0.007% achieved the best tenderizing effect on beef, primarily due to papain promoting the cleavage of Z-lines, resulting in a significant increase in the muscle fiber fragment index (MFI) and a significant decrease in shear force with prolonged storage time.

B. M. Naveena et al. [20] investigated the tenderizing effects of various plant proteases on water buffalo meat. The results indicated that water buffalo meat treated with papain exhibited a significant increase in myofibrillar protein solubility and a significant decrease in shear force values.
Due to its excellent protein hydrolyzing ability, papain can be applied in various fields. In marine fishing and low-value fish account for a significant proportion. If not properly utilized, this would result in substantial waste. We can use papain to produce concentrated hydrolyzed protein, which is superior to whole fish meat or fish protein concentrates, featuring good water solubility, low fat content, low ash content, and high protein content. Papain can also be used to prevent food browning and recover residual meat from animal bones during slaughter [21].
3.2 As a beer quality improver
Mainly used to improve beer quality. Papain can hydrolyze proteins in beer, partially hydrolyze some formed complexes, produce more peptides or amino acids, ensure high clarity of beer during freezing, and improve beer taste while optimizing the composition and ratio of original peptides and amino acids, effectively enhancing beer quality. According to reports, adding papain at a concentration of 0.08 mg/100 mL to beer during freezing storage yields the best clarification effect, increasing free amino acid content, reducing turbidity by 68.75%, and increasing free amino acids such as threonine, valine, and arginine increased by 8.2 times, 0.2 times, and 1.1 times, respectively [22].
3.3 As a biscuit softener
The use of papain in cookies and pastries can break down thiols, reduce dough gluten strength, improve cookie crumb structure, enhance crumb texture, and reduce defect rates while increasing yield; additionally, it can reduce the use of fats and sugars. It is suitable for the production of high-, medium-, and low-grade cookies, pastries, and bread with various flavors [23].

3.4 Used in seasoning production
Brewer's yeast is a by-product of the beer industry. Through the action of papain, aromatic yeast extract with a rich flavor is extracted, which can be seasoned to produce yeast seasoning sauce. This serves as an excellent seasoning, enhancing the value and utilization of brewer's yeast.
3.5 Used in the nutritional health product industry
Papain is primarily used in health supplements through two pathways: one is as a digestive aid, formulated into enteric-coated tablets for oral administration to alleviate digestive disorders; the other is to decompose plant and animal sources containing special proteins into various nutritional supplements. Fang Fuyong et al. [24] used papain to hydrolyze Bafei clam meat, and the results showed that the hydrolysate contained a high content of free amino acids, approximately 923.0 mg/100 ml (tryptophan not included), with essential amino acids accounting for 33.0%. Bafei clam meat, after being hydrolyzed with a composite protease and appropriately formulated, can be used to produce a nutrient-rich, seafood-flavored oral liquid with certain health benefits.
3.6 Application in Pet Food Production
Treating pet food with papain can reduce its viscosity, improve texture, and enhance flavor. Additionally, papain has anthelmintic properties, and papaya juice can be used to expel nematodes from the intestines of mammalian hosts.

Additionally, papain can be applied in the chemical and pharmaceutical industries. For example, adding papain to detergents like laundry powder can quickly remove bloodstains and sweat stains from clothing. Furthermore, papain can promote the effective extraction of traditional Chinese medicine components, assist in the identification of Rh blood types, and be used for auxiliary treatment of tumors.
4 Outlook
Currently, papain is widely used in the food industry, such as in the production of meat, beer, and seasonings. With the improvement of living standards in China, people are increasingly concerned about food safety and nutrition. Papain can be used to produce safe and nutritious foods, such as oral liquids with health benefits, which have a promising market outlook.
Additionally, to meet the growing demand for papain across various industries, particularly in the food and pharmaceutical sectors, researching methods to separate and purify papain with low cost and high purity is highly significant. Therefore, conducting in-depth studies on the extraction and separation purification technology of papain to obtain high-quality papain holds important practical value.
References
[1] Bernard P. OH, Andrew M. H, David J. B, et al. Crystal structure of glycyl endopeptidase from Carica papaya: a cysteine endopeptidase of unusual substrate specificity. Biochemistry, 1995, 34: 13190-13195.
[2] Yi Yin, Tan Aijuan, Liu Ning, et al. Study on the production process of papain [J]. Guizhou Agricultural Sciences, 2000, 28(5): 24-25.
[3] Tan Jing, Chen Jiwang, Xia Wenshui et al. Ultrafiltration separation of papain with chitinase activity [J]. Food and Machinery, 2007, 23(6): 20-23.
[4] Wang Libin, Zhang Tao, Wang Min. Separation, Purification, and Characterization of High-Purity Papain [J]. Chinese Journal of Biochemical Drugs, 2006, 27(3): 159-162.
[5] Mohamed A., Anouar E. M., Delphine V. W., et al. Fractionation and purification of the enzymes stored in the latex of Carica papaya [J]. Journal of Chromatography B, 2003, 790: 229-238.
[6] He Jiqin, Zhang Haide. Separation methods and applications of papain [J]. Food Science and Technology, 2006, 10: 66-69.
[7] KN OR RD, ZEN KER M, HEINZ V, et al. Applications and potential of ultrasonic in food processing [J]. Trends in Food Science and Technology, 2004, 15: 261-266.
[8] Wang Jing, Han Tao, Li Liping. Biological Effects of Ultrasound and Its Applications in the Food Industry [J]. Journal of Beijing Agricultural College, 2006, 21(1): 67-75.
[9] Xiao Guiping. Ultrasonic Extraction Process and Enzymatic Properties of Papain. Journal of Fujian Agricultural and Forestry University, 2005, 34(3):318-323.
[10] Nie Huali, Chen Tianxiang, Zhu Limin. Preparation of nylon affinity membranes and their separation and purification of papain [J]. Membrane Science and Technology, 2008, 28(1): 16-20.
[11] Guo W., Ruckenstein E. Separation and purification of horseradish peroxidase by membrane affinity chromatography [J]. Membrane Science, 2003, 211: 101-111.
[12] Sarote N., Rajni H. K., Pawinee K. Purification of papain from Carica papaya latex: Aqueous two-phase extraction versus two-step salt precipitation [J]. Enzyme and Microbial Technology, 2006, (39)5: 1103-1107.
[13] Fang Huan, Gao Xiangyang, Zhang Hongli et al. Study on the immobilization of papain on porous ceramic membranes [J]. Journal of South China Agricultural University, 2006, 27(4): 36-39.
[14] Gao Mingxia, Miao Jingzhi, Cao Zhehong et al. Study on the extraction of burdock polysaccharides using chitin-immobilized papain [J]. Food Science, 2007, 28(9): 226-229.
[15] Jin Feng. Study on microporous starch-sodium alginate immobilized papain. Chinese Brewing, 2009, (7): 83-85.
[16] Zou Zechang, Wei Qi, Na Wei et al. Study on silica mesoporous foam materials immobilized papain. Journal of Inorganic Materials, 2009, 24(4): 702-706.
[17] Lin Yin, Fang Run, Cheng Xiansu. Study on the adsorption of three proteases by lignin derivatives [J]. Journal of Biomass Chemical Engineering, 2009, 43(2): 6-10.
[18] Cai Xiaowen, Han Luqi, Jiang Qian Yong. Study on tenderizing powder (papain) [J]. Meat Research, 2005 (10): 42-44.
[19] Lei Changgui, Meng Yuzhu, Xi Huiping. Effects of calcium chloride, phosphates, and papain on MFI and shear force of beef [J]. Meat Industry, 2009, 2: 23-25.
[20] B.M. Naveena, S.K. Mendiratta, A.S.R. Anjaneyulu. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingibero cinale roscoe (Ginger rhizome) [J]. Meat Science, 2004, 68: 363-369.
[21] Yang Sang, Deng Shangui, Qin Xiaoming. Preparation and antioxidant and antibacterial activities of low-value fish protein peptides-calcium chelates [J]. Food Science, 2008, 29(1): 202-206.
[22] Shen Yue. Review of papain research and applications [J]. Science and Technology Information, 2008, 11: 313-314.
[23] Xiong Qiongchao, Xu Dechang. Advances in the application of papain [J]. China Sugar Beet Industry, 2008, (1): 43-45.
[24] Fang Fuyong, Miao Yanli, Song Wending. Development of a Hydrolysis Process for a Composite Enzyme from Wavy-Patterned Bivalve Shells and the Formulation of a Functional Oral Liquid [J]. Food Science, 2009, (18): 412-415.
-
Prev
Clean Label Alternative: Naturally Fermented Raspberry Ketone Ingredient
-
Next
What Is Papain and Its Uses in the Feed Industry?


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese





